Abstract
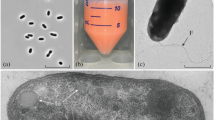
A novel group of hyperthermophilic rod-shaped motile methanogens was isolated from a hydrothermally heated deep sea sediment (Guaymas Basin, Gulf of California) and from a shallow marine hydrothermal system (Kolbeinsey ridge, Iceland). The grew between 84 and 110°C (opt: 98°C) and from 0.2% to 4% NaCl (opt. 2%) and pH 5.5 to 7 (opt: 6.5). The isolates were obligate chemolithoautotrophes using H2/CO2 as energy and carbon sources. In the presence of sulfur, H2S was formed and cells tended to lyse. The cell wall consisted of a new type of pseudomurein containing ornithin in addition to lysine and no N-acetylglucosamine. The pseudomurein layer was covered by a detergent-sensitive protein surface layer. The core lipid consisted exclusively of phytanyl diether. The GC content of the DNA was 60 mol%. By 16S rRNA comparisons the new organisms were not related to any of the three methanogenic lineages. Based on the physiological and molecular properties of the new isolates, we describe here a new genus, which we name Methanopyrus (the “methane fire”). The type species is Methanopyrus kandleri (type strain: AV19; DSM 6324).
Similar content being viewed by others
References
Balch WE, Wolfe RS (1976) New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)dependent growth of Methanobacterium ruminantium in a pressurized atmosphere. Appl Environ Microbiol 32:781–791
Balch WE, Fox GE, Magrum LJ, Woese CR, Wolfe RS (1979) Methanogens: reevaluation of a unique biological group. Microbiol Rev 43:260–296
Brenner DJ (1973) Deoxyribonucleic acid reassociation in the taxonomy of enteric bacteria. Int J Syst Bacteriol 43:298–307
Burggraf S, Jannasch HW, Nicolaus B, Stetter KO (1990a) Archaeoglobus profundus sp. nov., represents a new species within the sulfate-reducing archaebacteria. System Appl Microbiol 13:24–28
Burggraf S, Fricke H, Neuner A, Kristjansson JK, Rouvier P, Mandelco L, Woese CR, Stetter KO (1990b) Methanococcus igneus sp. nov., a novel hyperthermophilic methanogen from a shallow submarine hydrothermal system. Syst Appl Microbiol 13:263–269
De Rosa M, Gambacorta A, Nicolaus B, Chappe B, Albrecht P (1983) Isoprenoid ethers: backbone of complex lipids of the archaebacterium Sulfolobus solfataricus. Biochim Biophys Acta 753:249–256
Drobner E, Huber H, Wächtershäuser G, Rose D, Stetter KO (1990) Pyrite formation linked with hydrogen evolution under anaerobic conditions. Nature 346:742–744
Grunstein M, Hogness DS (1975) Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci USA 72:3961–3965
Hensel R, König H (1988) Thermoadaption of methanogenic bacteria by intracellular ion concentration. FEMS Microbiol Lett 49:75–79
Holt SC, Leadbetter ER (1967) Fine structure of Sporocytophaga myxococcoides. Arch Mikrobiol 57:199–213
Huber H, Thomm M, König H, Thies G, Stetter KO (1982) Methanococcus thermolithotrophicus, a novel thermophilic lithotrophic methanogen. Arch Microbiol 132:47–50
Huber R, Kurr M, Jannasch HW, Stetter KO (1989a) A novel group of abyssal methanogenic archaebacteria (Methanopyrus) growing at 110°C. Nature 342:833–834
Huber R, Woese CR, Langworthy TA, Fricke H, Stetter KO (1989b) Thermosipho africanus gen. nov., represents a new genus of thermophilic eubacteria within the “Thermotogales”. Syst Appl Microbiol 12:32–37
Huber R, Woese CR, Langworthy TA, Kristjansson JK, Stetter KO (1990) Fervidobacterium islandicum sp. nov., a new extremely thermophilic eubacterium belonging to the “Thermotogales”. Arch Microbiol 154:105–111
Humphries P, McConell DJ, Gordon RL (1973) A procedure for the purification of Escherichia coli deoxyribonucleic acid dependent ribonucleic acid polymerase. Biochem J 133:201–203
Jones WJ, Leigh JA, Mayer F, Woese CR, Wolfe RS (1983) Methanococcus jannaschii sp. nov., an extremely thermophilic methanogen from a submarine hydrothermal vent. Arch Microbiol 136:154–261
Kandler O, König H (1978) Chemical composition of the peptidogylcan-free cell walls of methanogenic bacteria. Arch Microbiol 118:141–152
Kelly RB, Cozzarelli NR, Deutscher MP, Lehmann JR, Kornberg A (1970) Enzymatic synthesis of deoxyribonucleic acid. XXXII. Replication of duplex deoxyribonucleic acid by polymerase at a single strand break. J Biol Chem 245:39–45
König H, Kandler O (1979a) The amino acid sequence of the peptide moiety of the pseudomurein of Methanobacterium thermoautotrophicum. Arch Microbiol 121:271–275
König H, Kandler O (1979b) N-Acetyltalosaminuronic acid, a constituent of the pseudomurein of the genus Methanobacterium. Arch Microbiol 123:295–299
König H, Kralik R, Kandler O (1982) Structure and modifications of pseudomurein of Methanobacteriales. Zentralbl Bakt Hyg I. Abt Orig C 3:179–191
König H, Kandler P, Jensen M, Rietschel ETh (1983) The primary structure of the glycan moiety of the pseudomurein from Methanobacterium thermoautotrophicum. Hoppe-Seyler's Z Physiol Chem 364:627–636
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Langworthy TA, Pond JL (1986) Membranes and lipids of thermophiles. In: Brock TD (ed) Thermophiles: general, molecular, and applied microbiology. Wiley, New York, pp 107–135
Lauerer G, Kristiansson JK, Langworthy TA, König H, Stetter KO (1986) Methanothermus sociabilis sp. nov., a second species within the Methanothermaceae growing at 97°C. Syst Appl Microbiol 8:100–105
Madon J, Zillig W (1983) A form of the DNA-dependent RNA polymerase of Halobacterium halobium, containing an additional component, is able to transcribe native DNA. Eur J Biochem 133:471–474
Marmur J, Doty P (1961) Thermal renaturation of deoxyribonucleic acids. J Mol Biol 3:585–594
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperatures. J Mol Biol 5:109–118
Meyer SA, Schleifer KH (1978) Deoxyribonucleic acid reassociation in the classification of coagulase-positive staphylococci. Arch Microbiol 117:183–188
Mirault ME, Scherrer K (1971) Isolation of preribosomes from Hela cells and their characterization by electrophoresis on uniform and exponential-gradient-polyacrylamide gels. Eur J Biochem 23:372–386
Pfennig N, Wagener S (1986) An improved method of preparing wet mounts for photomicrographs of microorganisms. J Microbiol Methods 4:303–306
Rudnick HS, Hendrich S, Pilatus U, Blotevogel HK (1990) Phosphate accumulation and the occurrence of polyphosphates and cyclic 2,3-diphosphoglycerate in Methanosarcina frisia. Arch Microbiol 154:584–588
Schnabel R, Thomm M, Gerardy-Schahn R, Zillig W, Stetter KO, Huet J (1983) Structural homology between different archaebacterial DNA-dependent RNA polymerases analyzed by immunological comparison of their components. EMBO J 2:751–755
Seely RJ, Fahrney DE (1984) The cyclic 2,3-diphosphoglycerate from Methanobacterium thermoautotrophicum is the d-enantiomer. Curr Microbiol 10:85–88
Stetter KO (1982) Ultrathin mycelia-forming organisms from submarine vulcanic areas having an optimum growth temperature of 105°C. Nature 300:258–260
Stetter KO, König H, Stackebrandt E (1983) Pyrodictium gen. nov., a new genus of submarine disc-shaped sulphur reducing archaebacteria growing optimally at 105°C. Syst Appl Microbiol 4:535–551
Stetter KO, Thomm M, Winter J, Wildgruber G, Huber H, Zillig W, Janecovic D, König H, Palm P, Wunderl S (1981) Methanothermus fervidus, sp. nov., a novel extremely thermophilic methanogen isolated from an Icelandic hot spring. Zentralbl Bakt Hyg I Orig C 2:166–178
Stetter KO, Fiala G, Huber G, Huber R, Segerer A (1990) Hyperthermophilic microorganisms. FEMS Microbiol Rev 75:117–124
Thomm M, Madon J, Stetter KO (1986) DNA-dependent RNA polymerase of the three orders of methanogens. Biol Chem Hoppe-Seyler 367:473–481
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: Procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Trincone A, De Rosa M, Gambacorta A, Lanzotti V, Nicolaus B, Harris JE, Grant WD (1988) A simple chromatographic procedure for the detection of cyclized archaebacterial glycerol-bisphytanyl-glycerol tetraether core lipids. J Gen Microbiol 134:3159–3163
Woese CR, Kandler O, Wheelis ML (1990) Towards a natural system of organisms: Proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci USA 87:4576–4579
Zeikus JG, Wolfe RS (1972) Methanobacterium thermoautotrophicum sp. nov., an anaerobic, autotrophic, extreme thermophile. J Bacteriol 109:707–713
Zillig W, Stetter KO, Wunderl S, Schulz W, Priess H, Scholz J (1980) The “Sulfolobus-Caldariella”-group: Taxonomy on the basis of the structure of DNA-dependent RNA polymerases. Arch Microbiol 125:259–269
Zobell CE (1941) Studies on marine bacteria. I. The cultural requirements of heterotrophic aerobes. J Marin Res 4:42–75
Author information
Authors and Affiliations
Additional information
This paper is dedicated to Otto Kandler on the occasion of his 70th birthday
Rights and permissions
About this article
Cite this article
Kurr, M., Huber, R., König, H. et al. Methanopyrus kandleri, gen. and sp. nov. represents a novel group of hyperthermophilic methanogens, growing at 110°C. Arch. Microbiol. 156, 239–247 (1991). https://doi.org/10.1007/BF00262992
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00262992