Abstract
For more than half a century, low-density lipoprotein cholesterol (LDL-C) has been recognized as a major risk factor for incident atherosclerotic cardiovascular disease. The discovery of proprotein convertase subtilisin-kexin type 9 (PCSK9) in 2003, which prevents LDL-C receptor recycling, identified a new _target for drug intervention. Recently, two large-scale randomized clinical outcomes trials involving fully human anti-PCSK9 monoclonal antibodies tested the hypothesis that _targeting this pathway would reduce cardiovascular events. Both the FOURIER (Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk) and ODYSSEY OUTCOMES trials met their primary efficacy endpoints, confirming findings reported earlier that major adverse cardiovascular events can be reduced by a further lowering of LDL-C beyond that achieved with statin therapy. In both trials, there were incremental reductions in LDL-C of > 50% from baseline, with no major safety concerns, over the trials’ median follow-up time (2.2 and 2.8 years, respectively). While there were differences in design, lipid management and overall results, key messages from both studies were similar. However, post-publication, additional questions have arisen, especially regarding drug effects over the long-term, including a potential mortality benefit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
LDL-cholesterol (LDL-C) is causally associated with incident atherosclerotic cardiovascular (CV) disease. |
While statins have been demonstrated to significantly reduce CV events, residual risk remains, especially among patients with prior atherosclerotic CV disease or ischemic events. |
Proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors provide a new option for patients who do not achieve optimal levels of LDL-C with statins. |
Two pivotal CV outcomes trials, the FOURIER and ODYSSEY OUTCOMES studies, have demonstrated that two monoclonal antibodies against PCSK9 reduced major adverse CV events in high-risk patients. |
However, some questions remain to be answered, such as the long-term safety, cost-effectiveness, reduction in mortality and whether patients with lower risk profiles may also benefit from this intense LDL-C lowering therapy on top of statins. |
Introduction
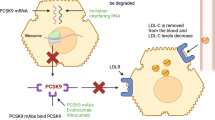
It is well-known that low-density lipoprotein cholesterol (LDL-C) is a major modifiable risk factor involved in atherosclerotic heart disease. The Cholesterol Treatment Trialists (CTT) meta-analysis demonstrated that risk reductions for major adverse cardiovascular events (MACE) with statin therapy have a consistent relationship with the absolute reduction in LDL-C [1]. Subsequently, proprotein convertase subtilisin–kexin type 9 (PCSK9) inhibitors were developed as a novel treatment option in modern lipidology [2, 3]. Since the discovery that loss-of-function mutations of this protein are associated with a lower incidence of coronary heart disease (CHD) [4], new drugs _targeting PCSK9 have been sought. The recent publication of two large-scale randomized cardiovascular (CV) outcomes trials with anti-PCSK9 monoclonal antibodies brought the use of these drugs in LDL-C-lowering therapy a step further [5, 6]. While there were some differences in terms of study design between these two large trials, the clinical implications are consistent: inhibition of PCSK9 decreases MACE over a medium-term follow-up (< 3 years), with no major safety issues, including in patients who have already achieved very low LDL-C levels. In addition, in both trials the relative risk reduction in CV events per milimole/liter LDL-C lowering is consistent with that achieved with other lipid-lowering therapies, including statins [1] and ezetimibe [7].
The main aim of this review was to compare study designs and key findings; critically analyze clinical implications; and discuss gaps in knowledge that remain to be addressed in future studies with PCSK9 inhibitors.
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Designs and Baseline Characteristics of the Fourier and Odyssey Outcomes Trials
The FOURIER (Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk) trial tested the fully human monoclonal antibody evolocumab at either 140 mg every 2 weeks (Q2W) or 420 mg every 4 weeks (Q4W) given by subcutaneous (SC) injection (these are considered to be equipotent regimens) [8]. The ODYSSEY OUTCOMES trial tested another fully human monoclonal antibody, alirocumab, at an initial dose of 75 mg Q2W (with a protocol-driven up-titration to 150 mg when LDL-C was > 50 mg/dL), also by SC injection. From a pharmacological point of view, both drugs were well tolerated in phase 2 studies and led to a consistent decrease in LDL-C as well as a decrease in apolipoprotein B (ApoB) and lipoprotein (a) [9, 10].
The design [11] and results [5] of the FOURIER trial have been published. The main inclusion criteria were age between 40 and 85 years plus prior established CV disease, which included either previous non-hemorrhagic stroke, myocardial infarction (MI) or symptomatic peripheral artery disease (PAD). Patients were required to have a baseline LDL-C ≥ 70 mg/dL or non-high-density cholesterol (HDL-C) ≥ 100 mg/dL on stable treatment with at least moderate-intensity (preferentially high-intensity) statin, with or without ezetimibe. In addition, the patients were required to have at least one additional risk criterion, such as diabetes mellitus (DM), age ≥ 65 years or more than one prior MI/stroke or qualifying MI/stroke having occurred ≤ 6 months before randomization (see Table 1 for further details).
In the ODYSSEY OUTCOMES trial, qualifying patients were at least 40 years of age, hospitalized with an acute coronary syndrome (ACS) between 4 and 52 weeks before randomization and had a baseline LDL-C ≥ 70 mg/dL or non-HDL-c ≥ 100 mg/dL or ApoB ≥ 80 mg/dL. Unlike the FOURIER trial, there was no need for an incremental risk factor besides the index ACS. In addition, a high-intensity statin regimen with the maximum tolerated dose was required as background therapy [6, 12] (Table 1).
Comparison of Trial Designs
Lipid enrollment criteria were similar in the FOURIER and ODYSSEY OUTCOMES trials, with both studies requiring eligible subjects to have LDL-C ≥ 70 mg/dL or non-HDL-C ≥ 100 mg/dL while on baseline statin therapy (or other allowed lipid-lowering drugs, if statins were not tolerated; see below for further details). In the ODYSSEY OUTCOMES trial, only 132 patients (0.7%) qualified based solely on the ApoB ≥ 80 mg/dL criterion. Recent ACS was an exclusion criterion in the FOURIER trial, whereas it was a major inclusion criterion in the ODYSSEY OUTCOMES trial. Regarding other exclusion criteria, both trials were very similar, with no major differences (Table 2).
In the FOURIER trial, more than three-quarters of enrolled patients had a history of prior MI (median time from index-event 3.4 years), 19% had a prior non-hemorrhagic stroke and 13% had PAD. PAD, similar to prior MI and stroke, is also well recognized as a major incremental risk factor as it is a marker of more widespread atherosclerosis [13,14,15]. The ODYSSEY OUTCOMES trial, on the other hand, _targeted a more acute population—patients with recent ACS. This same group has been previously addressed in other lipid-lowering trials [7, 16,17,18]. One remarkable overlap between both trials was the number of patients with prior MI (83% in ODYSSEY OUTCOMES vs. 81% in FOURIER), thus highlighting the fact that both trials indeed enrolled high-risk groups with established CAD (Table 3).
Fourier Versus Odyssey Outcomes trials: Baseline Lipid-Lowering Therapy and Management of LDL-C Levels
Considering the accumulated evidence from studies on statins, especially those with high-intensity regimens [1, 16, 17, 19], achieving further clinical benefit on top of this standard of care might appear challenging. Both study protocols strived to identify patients who were optimally treated before enrollment. The FOURIER trial required patients to be on moderate or high-intensity statin therapy, such as, for example, at least atorvastatin 20 mg daily or its equivalent, while the ODYSSEY OUTCOMES trial required patients to be on atorvastatin 40–80 mg or rosuvastatin 20–40 mg daily, or the maximum tolerated dose of one of these two agents. Therefore, the vast majority of patients were treated at baseline with high-intensity statins (70% in FOURIER and 92% in ODYSSEY OUTCOMES). Ezetimibe was used infrequently at baseline (3–5% of patients in both trials). Nonetheless, the median baseline LDL-C levels in both trials were similar (92 mg/dL in FOURIER and 87 mg/dL in ODYSSEY OUTCOMES; see Table 3).
In both trials, special care was undertaken regarding background lipid-lowering therapy and how lipid levels were monitored during the studies. Lipid levels were not disclosed to either the investigators or patients in order to avoid unblinding and minimize the introduction of bias and confounding. In both trials, changes in background lipid-lowering therapies were discouraged after randomization. Moreover, in the FOURIER trial there was an independent lipid monitoring committee whose role was to monitor the LDL-C separation between treatment groups (but not clinical events) over the course of the study. Their charge was to periodically review unblinded LDL-C data and to advise the Executive Committee and sponsor if trial assumptions were not being met globally or in certain subgroups. In the ODYSSEY OUTCOMES trial there was an independent and unblinded committee dedicated to monitor safety in patients with low LDL-C values (< 25 mg/dL) whose members reported individual and aggregate findings to the Data Safety Monitoring Board (DSMB). Due to previous concerns of a possible association of lipid lowering with hemorrhagic stroke [20] and given that the majority of patients would be expected to be on dual antiplatelet therapy in the ODYSSEY OUTCOMES trial (enrolling 1 year post-ACS population), LDL-C levels of < 15 mg/dL were considered to be undesirable due to a potential increased risk of intracranial hemorrhage. Thus, the protocol mandated permanent study drug discontinuation when LDL-C levels < 15 mg/dL were sustained while on the lower dose (see below).
The major difference in lipid management between the studies was the titration of PCSK9 inhibitor therapy in the ODYSSEY OUTCOMES trial design. All patients assigned to the active alirocumab arm were started on alirocumab 75 mg Q2W, which is half of the maximally approved dose. If LDL-C levels at 1 month after randomization were > 50 mg/dL, study drug dose was increased to 150 mg Q2W in a blinded fashion. On the other hand, if LDL-C levels dropped to < 25 mg/dL, patients on the 150 mg dose had their dose blindly halved, while patients on 75 mg were maintained at this dose. However, if levels of < 15 mg/dL were determined in two consecutive measurements while on the 75 mg dose, then the patient was blindly switched to placebo. If patients had LDL-C values of between 15 and 25 mg/dL and had an adverse event that was considered to be causally related to the treatment by the study safety physician, then they were also blindly switched to placebo. During the trial, 2615 of the patients in the alirocumab arm (27.6%) had their therapies up-titrated to the 150 mg dose after initial assessment of LDL-C levels, whereas 730 (7.7%) patients were switched in a blinded fashion to placebo due to consecutive measured LDL-C levels of < 15 mg/day on the 75 mg dose.
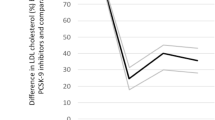
Both trials achieved a > 50% reduction in LDL levels (see Table 3). Accordingly, achieved LDL-C levels were lower in the FOURIER trial than in the ODYSSEY OUTCOMES trial despite the baseline mean LDL-C levels being similar in both trials. In the FOURIER trial, mean differences in LDL-C levels between treatment arms at 12 and 24 months were 56 and 54 mg/dL, respectively; in the ODYSSEY OUTCOMES trial, mean differences at 12 and 24 months were 48 and 44 mg/dL, respectively. In addition to study drug discontinuation and blindly switching to placebo, down-titration of background statin therapy may have contributed to the increase in LDL-C over time and, even if more common in the alirocumab arm, may have contributed to a blunting of the difference between the two randomized groups in the ODYSSEY OUTCOMES trial (Fig. 1).
Despite prior concerns regarding a possible association between high-intensity statin therapy, low achieved LDL-C, and hemorrhagic stroke [20], further data on this topic has been reassuring. In a pre-specified secondary analysis from the FOURIER trial, the 2669 patients who achieved LDL-C < 20 mg/dL had no major safety concerns (including no increase in risk of hemorrhagic stroke) [21]. In a similar analysis from the IMPROVE-IT (testing ezetimibe vs. placebo in addition to simvastatin) study, the 971 patients with achieved LDL-C of < 30 mg/dL at 1 month had no excess safety concerns, including hemorrhagic stroke, over a median follow-up of 6 years [22].
Study Designs: Efficacy Endpoints
In the FOURIER trial, the primary efficacy endpoint (PEP) was the composite of CV death, MI, any stroke, hospitalization for unstable angina or unplanned coronary revascularization, and the key secondary endpoint was the composite of CV death, MI or any stroke. In the ODYSSEY OUTCOMES trial, the primary endpoint was the composite of CHD death, MI, ischemic stroke or hospitalization for UA, and the secondary endpoint was the composite of all-cause death, MI or ischemic stroke.
Some differences in the aforementioned primary endpoints for each trial should be emphasized (see Table 1). CV death in the FOURIER trial was defined as death resulting from an acute MI, sudden cardiac death, heart failure (HF), stroke, CV procedures, CV hemorrhage and other CV causes. CHD death in the ODYSSEY OUTCOMES trial was defined as any death with a clear relationship to underlying CHD, including death secondary to acute MI, sudden death, HF or complications arising from a coronary revascularization procedure where the cause of death was clearly related to the procedure. Therefore, CV death comprises a broader endpoint that also includes deaths due to non-coronary CV disease (e.g., arrhythmia, pulmonary embolism, hemorrhagic stroke), whereas CHD death includes only those deaths directly or presumably attributable to CAD and its complications. In the FOURIER trial the primary endpoint included all types of stroke, whereas the primary endpoint in the ODYSSEY OUTCOMES trial included only ischemic strokes; thus, hemorrhagic strokes and those of uncertain origin were part of the PEP only in the FOURIER trial. In the FOURIER trial the revascularization endpoint was defined as any unplanned revascularization, and in the ODYSSEY OUTCOMES trial only ischemia-driven revascularization (prompted by ischemic symptoms or abnormal findings in functional tests) was included. Of note, coronary stent re-stenosis was excluded from the outcome definitions in the ODYSSEY OUTCOMES trial since it was considered less amenable to modification by therapy aimed at lowering LDL-C [23], while those events were counted in the PEP of the FOURIER trial.
Fourier Versus Odyssey Outcomes: Overall Results
Both trials met their respective PEP, with significant reductions favoring the PCSK9 inhibitor compared to placebo. The point estimates of the hazard ratios (HRs) for the PEPs were similar in the FOURIER and ODYSSEY OUTCOMES trials (HR 0.85, 95% confidence interval [CI] 0.79–0.92 and HR 0.85, 95% CI 0.78–0.93, respectively) (Fig. 2). There was also a consistent benefit from both active drugs regarding reductions in MI (FOURIER: HR 0.73, 95% CI 0.65–0.82; ODYSSEY OUTCOMES: HR 0.86, 95% CI 0.77–0.96) and stroke (FOURIER: HR 0.79, 95% CI 0.66–0.95; ODYSSEY OUTCOMES: HR 0.73, 95% CI 0.57–0.93). The revascularization endpoints from the FOURIER and ODYSSEY OUTCOMES trials were both significantly reduced, but with numerically different point estimates, possibly related to differences in patient characteristics in each trial (post-ACS vs. stable CAD) and/or different definitions used for the revascularization endpoint. Regarding CV death, neither trial demonstrated a statistically significant benefit of the PCSK9 inhibitor over placebo, although in the ODYSSEY OUTCOMES trial there was a lower rate of all-cause death favoring alirocumab that did not meet the rigorous statistical criteria for significance specified in the protocol to correct for multiplicity. This issue is explored further in a subsequent section.
Comparison of the ODYSSEY and FOURIER trials in terms of clinical efficacy based on the hazard ratios (HR) for the primary and secondary endpoints. HR is presented with the 95% confidence interval (CI). For the FOURIER trial, myocardial infarction (MI) included both fatal and non-fatal events; for the ODYSSEY trial, MI included only non-fatal events. Stroke in the FOURIER trial considered all strokes, whereas in the ODYSSEY OUTCOMES trials only ischemic strokes were taken into account. The definition of coronary revascularization endpoint in the FOURIER trial comprised any revascularization procedure, whereas only ischemia-driven revascularization was included in this endpoint in the ODYSSEY OUTCOMES trial. Superscript 1 indicates coronary death, MI, ischemic stroke or unstable angina in the ODYSSEY Outcomes trial and cardiovascular death, MI, stroke, unstable angina or coronary revascularization in the FOURIER trial. Superscript 2 indicates all-cause death, MI or ischemic stroke in the ODYSSEY Outcomes trial and cardiovascular death, MI or stroke in the FOURIER trial. Superscript (3) indicates that MI includes fatal and non-fatal events in the FOURIER trial and only non-fatal eventsin the ODYSSEY trial . Stroke in the FOURIER trial explicitly refers to all strokes, whereas in the ODYSSEY OUTCOMES trials it refers to only ischemic strokes. Superscript 4 refers to all types of stroke in the FOURIER study and only ischemic stroke in the ODYSSEY Outcomes trial
Does Baseline LDL-C Matter?
A meta-analysis carried out by the CTT Collaboration showed that irrespective of the baseline LDL-C level, clinical benefit was most strongly related to the absolute reduction in LDL-C [1]. The 2018 Multisociety Guideline on cholesterol treatment continues to recommend an initial percentage LDL-C lowering (at least 50% from baseline), but it has also introduced a threshold of 70 mg/dL in high-risk patients before considering the addition of other lipid-lowering drugs to the statin therapeutic regimen [24].
In the FOURIER trial, there was no significant heterogeneity of treatment effect according to quartiles of baseline LDL-C level (< 80, 80 to < 92, 92–109 and > 109 mg/dL), such that the benefit was consistent across those subgroups. A dedicated secondary analysis of the FOURIER trial data showed similar clinical benefits with the addition of evolocumab to the treatment regimen among patients on maximum intensity statin therapy (atorvastatin 80 mg or rosuvastatin 40 mg) versus those on submaximal statin therapy, and in patients with baseline LDL-C levels < 70 mg/dL versus those with baseline LDL-C levels ≥ 70 mg/dL (and considered to be below the treatment threshold) [25]. Moreover, a secondary analysis of FOURIER trial data demonstrated a persistence of benefit even at achieved levels as low as < 20 mg/dL [21].
In comparison, in the ODYSSEY trial, a trend for interaction between treatment and baseline LDL-C was demonstrated, favoring a greater relative risk reduction in patients with baseline LDL-C > 100 mg/dL. This result is in accordance with findings reported earlier from a meta-analysis showing a reduction in events proportional to the absolute change in LDL-C with different pharmacological therapies [26]. If patients are started on therapy at a higher baseline LDL-C, then a greater absolute reduction in LDL-C level is expected, translating into larger reductions in clinical events. However, data from both trials support the notion that patients with lower levels of LDL-C still have benefit from therapy with PCSK9 inhibitors (with relative risk reductions in PEP of 14 and 20% for patients with baseline LDL-C < 80 mg/dL in the ODYSSEY OUTCOMES and FOURIER trials, respectively). Also, it is important to note that in the ODYSSEY OUTCOMES trial, patients with baseline LDL-C levels > 100 mg/dL would be more likely to have the dose of alirocumab up-titrated to 150 mg, whereas patients with lower baseline LDL would be more likely to be maintained on alirocumab 75 mg, thereby leading to even greater absolute LDL-C lowering and potentially a larger reduction in clinical events in the subgroup with higher baseline LDL-C levels. In addition, patients with lower baseline LDL-C levels were more likely to have been switched to placebo in the active arm due to consecutive on-treatment LDL-C levels of < 15 mg/dL, thus driving the benefit of the alirocumab arm towards the null in that subgroup.
Do PCSK9 Inhibitors Provide Mortality Reduction?
Taking into account that atherosclerosis is a long-standing disease whose consequences take years to ensue, the aim of lipid-lowering therapy is to postpone or halt the progression of this disease. As such, mortality benefits might be anticipated only after several years have elapsed. Not surprisingly, CV death has not been consistently reduced in modern lipid-lowering trials comparing high-intensity with lower-intensity lipid-lowering therapies [7, 16, 18,19,20, 27, 28]. Moreover, although trials comparing statin therapy with placebo reported a significant 12% reduction in all-cause mortality at 5 years in the pooled trials result, there was a significant time-dependent effect, with no benefit demonstrated before the first 1–2 years [29]. If one assumes no benefit in CV mortality during the first 1.5 years of treatment with PCSK9 inhibitor and considers the relatively short follow-up in FOURIER and ODYSSEY OUTCOMES trials, then the expected relative risk reduction in CV death for both trials would be approximately 6% [30]. This estimate is similar to that reported in a recent meta-analysis of 39 randomized trials with PCSK9 inhibitors [31].
Can it be Demonstrated that PCSK9 Inhibitors Provide Mortality Reduction?
In the FOURIER trial, there was no sign of a reduction in CV or all-cause death in the evolocumab arm. The same was true for the SPIRE-1 and SPIRE-2 (Studies of PCSK9 Inhibition and the Reduction of Vascular Events) trials with bococizumab, although these two trials were discontinued prematurely (after a median follow-up of 7 and 12 months, respectively) due to the appearance of neutralizing antidrug antibodies [32]. In the ODYSSEY OUTCOMES trial, all-cause death was lower in the alirocumab arm, with a HR of 0.85 (95% CI 0.73–0.98), albeit the difference between treatment arms was not considered to be statistically significant after adjustment for multiple comparisons. The neutral effect of alirocumab on CHD deaths (HR 0.92, 95% CI 0.76–1.11) weakens the biological plausibility of this finding. Furthermore, a secondary analysis of causes of death in the ODYSSEY OUTCOMES trial revealed a numerically greater risk reduction in non-CV deaths (HR 0.77, 95% CI 0.59–1.01) than in CV deaths (HR 0.88, 95% CI 0.74–1.05) with alirocumab. Among the 27 fewer non-CV deaths prevented by alirocumab, the largest difference was for deaths due to pulmonary causes [33]. The only reasonable explanations for the larger reduction in non-CV deaths with alirocumab would appear to be: (1) misclassification of the causes of death; (2) another unknown pleiotropic effect of this drug on non-CV deaths that remains to be demonstrated; (3) chance. The first explanation alone seems implausible as one would have to assume that the treatment effect of the “misclassified” deaths in the non-CV death group was substantially greater than the treatment effect in the group with adjudicated CV causes of death.
Nevertheless, when considering that PCSK9 inhibitors reduced MACE with no major safety concern within the time limits of both trials, it seems reasonable to expect a significant reduction in fatal events also with longer follow-up. Two ongoing studies with PCSK9 inhibitors, ORION-4 (NCT03705234), with inclisiran (a small interfering RNA administered every 6 months), and VESALIUS-CV (NCT03872401), with evolocumab, in patients at high-risk of CV event without prior MI or stroke followed for 4–5 years should help to clarify these issues.
Safety Issues
Overall, both drugs were well tolerated, with no major safety concerns in either trial (Fig. 3). In the FOURIER trial, 1.6% of patients in the evolocumab group versus 1.5% in the placebo group had an adverse event thought to be related to the study drug and leading to drug discontinuation. In the ODYSSEY OUTCOMES trial, 3.6% of patients in the alirocumab group versus 3.4% in the placebo group had an adverse event leading to drug discontinuation. The only adverse event in both trials that was significantly higher in patients receiving active therapy than in those on placebo was local (and mostly minor) injection site reaction (2.1 vs. 1.6% in FOURIER and 3.8 vs. 2.1% in ODYSSEY OUTCOMES; see Fig. 3 for further details). One reassuring finding was that new-onset DM was not significantly increased with either of the PCSK9 inhibitors. This contrasts with results from prior Mendelian randomization studies and pre-clinical data suggesting that inhibition of the PCSK9 pathway could be associated with a modestly higher risk of new-onset DM [34,35,36]. In a pre-specified sub-analysis of data from the FOURIER trial, no differences in terms of adverse events (including new onset diabetes, cancer, intracranial bleeding and cognitive impairment) were demonstrated across five groups stratified according to achieved LDL-C at 4 weeks (< 20, 20–49, 50–69, 70–99 and ≥ 100 mg/dL) [21].
Comparison of the ODYSSEY and FOURIER trials in terms of safety. Main adverse events (AEs) are reported for each trial (percentage of patients with events). ALT Alanine aminotransferase, DM diabetes mellitus, pts patients, ULN upper limit of normal. For all AEs except injection site reactions, p > 0.05 for the comparison between the active drug arm versus placebo within each study
In prior randomized trials designed for LDL-C lowering, both evolocumab and alirocumab demonstrated a possible signal for higher rates of adverse events related to cognitive impairment [37, 38]. However, these prior findings were not confirmed in the ODYSSEY OUTCOMES and FOURIER trials. Adverse events related to cognition were similar between the active drug and placebo arms in both studies (1.6 vs. 1.5% in FOURIER and 1.5 vs. 1.8% in ODYSSEY OUTCOMES, respectively). Furthermore, within the FOURIER trial, a dedicated study known as EBBINGHAUS, with a shorter follow-up than that of the main trial (median 19 months), demonstrated no signal of harm on cognitive function based on evaluation by a structured standard battery of cognitive tests or in the pre-specified patient self-survey of everyday cognition [39]. An assessment is ongoing in a separate trial of alirocumab in patients with established CV disease or familial hypercholesterolemia using formal cognitive testing; these results are expected in 2020 (NCT02957682). While these data are reassuring, more information is still needed regarding long-term effects, since both trials had a median follow-up of < 3 years. To help further clarify this issue, there is an ongoing dedicated cognitive evaluation in approximately 500 patients from open-label studies with evolocumab (NCT 02867813); results are anticipated in 2021.
Final Considerations and Future Perspectives
The concept that lowering LDL-C reduces CV events, already well-established with statins and more recently established with other LDL-C lowering drugs [1, 7], has now been confirmed to extend to PCSK9 inhibitors, a new class of drugs that can reduce LDL-C to unprecedently low levels. Furthermore, the lack of major safety concerns with PCSK9 inhibitors found in both the FOURIER and ODYSSEY OUTCOMES trials is encouraging. However, several important questions remain to be answered.
First, the relatively high cost of these drugs compared to other lipid-lowering therapies remains an issue. Of note, the 2018 ACC/AHA Multisociety Guideline includes a cost-effectiveness evaluation in the shared decision-making algorithm for clinicians who are considering prescribing one of those drugs, thus placing PCSK9 inhibitors as the third choice after statins and ezetimibe [24]. This balance may change in the near future, given the recent cost reductions in alirocumab and evolocumab [40] and ongoing development of other, less expensive PCSK9 inhibitors [41].
A second consideration is the relative short-term follow-up in both trials. Since atherosclerosis is a degenerative progressive disease, data from life-long treatments aimed at halting its progression are needed. The GLAGOV randomized trial with evolocumab demonstrated atheroma plaque regression, as evaluated by intra-coronary ultrasound, in the relatively short term (76 weeks) [42]. Whether this will translate into the regression of angiographic stenosis with longer term therapy remains to be determined. From the safety perspective, absence of worrisome adverse reactions in short-term clinical trials does not rule out all possible safety issues. More data on the long-term effects associated with very low LDL-C levels over many years are needed, although neither genetic studies [4] nor evidence with evolocumab at 5 years [43] and ezetimibe at 6 years have shown harm [22]. In this context, post-market surveillance data and registries could provide additional relevant long-term safety data. Finally, open-label extension studies from previously published trials on PCSK9 inhibitors are underway and can also address potentially long-term benefits and at the same time rule out possible long-term harms (NCT 02867813, NCT03080935, NCT 01439880, NCT01954394, NCT01854918).
Finally, it remains to be established if and when mortality reduction can be achieved with PCSK9 inhibitors. This question is the most challenging one, since it would be more easily answered in a study enrolling very high-risk patients followed for > 4 years. However, because of the results just presented and the changes in guidelines, in order to keep equipoise, other populations or study designs are needed. Furthermore, given the declining mortality from MI in the developed world and the change in the pathophysiology of ACS in the statin era (with plaque erosions being more frequent than plaque rupture) [44], such studies would need to be larger and longer than previous studies in the field.
Altogether, the results from the FOURIER and ODYSSEY OUTCOMES trials underscore even more the notion that the PCSK9 protein is an important _target to reduce LDL-C and CV events. More than ever, both trials have reinforced the understanding that, in terms of LDL-C, the lower the better.
References
Cholesterol Treatment Trialists’ (CTT) Collaboration, Baigent C, Blackwell L, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010; 376:1670–81.
Seidah NG, Benjannet S, Wickham L, et al. The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): liver regeneration and neuronal differentiation. Proc Natl Acad Sci USA. 2003;100:928–33.
Abifadel M, Varret M, Rabès JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–6.
Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH. Sequence variations in PCSK9, low LDL, and protection against coronary heart disease. N Engl J Med. 2006;354:1264–72.
Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017; 376: 1713–22.
Schwartz GG, Steg PG, Szarek M, et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med 2018; 379:2097–2107.
Cannon CP, Blazing MA, Giugliano RP et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med 2015; 372:2387–97.
Wasserman SM, Sabatine MS, Koren MJ, et al. Comparison of LDL-C reduction using different evolocumab doses and intervals: biological insights and treatment implications. J Cardiovasc Pharmacol Ther. 2018;23:423–32.
McKenney JM, Koren MJ, Kereiakes DJ, Hanotin C, Ferrand AC, Stein EA. Safety and efficacy of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease, SAR236553/REGN727, in patients with primary hypercholesterolemia receiving ongoing stable atorvastatin therapy. J Am Coll Cardiol. 2012;59:2344–53.
Dias CS, Shaywitz AJ, Wasserman SM, et al. Effects of AMG 145 on low-density lipoprotein cholesterol levels: results from 2 randomized, double-blind, placebo-controlled, ascending-dose phase 1 studies in healthy volunteers and hypercholesterolemic subjects on statins. J Am Coll Cardiol. 2012;60:1888–98.
Sabatine MS, Giugliano RP, Keech A, et al. Rationale and design of the Further cardiovascular OUtcomes Research with PCSK9 Inhibition in subjects with Elevated Risk trial. Am Heart J. 2016;173:94–101.
Schwartz GG, Bessac L, Berdan LG, et al. Effect of alirocumab, a monoclonal antibody to PCSK9, on long-term cardiovascular outcomes following acute coronary syndromes: rationale and design of the ODYSSEY OUTCOMES trial. Am Heart J. 2014;168:682–9.
Bonaca MP, Nault P, Giugliano RP, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation. 2018;137:338–50.
Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67:2719–28.
Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet. 2018; 391:219–29.
Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004; 350:1495–504.
de Lemos JA, Blazing MA, Wiviott SD, Lewis EF, Fox KA, White HD, Rouleau JL, Pedersen TR, Gardner LH, Mukherjee R, Ramsey KE, Palmisano J, Bilheimer DW, Pfeffer MA, Califf RM, Braunwald E; Investigators. Early intensive vs delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004; 292:1307–16.
Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001; 285:1711–8.
LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med 2005; 352:1425–35.
Amarenco P, Bogousslavsky J, Callahan A 3rd, Goldstein LB, Hennerici M, Rudolph AE, Sillesen H, Simunovic L, Szarek M, Welch KM, Zivin JA; Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med 2006; 355:549–59.
Giugliano RP, Pedersen TR, Park JG, et al. Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet 2017; 390:1962–71.
Giugliano RP, Wiviott SD, Blazing MA, et al. Long-term safety and efficacy of achieving very low levels of low-density lipoprotein cholesterol: a prespecified analysis of the IMPROVE-IT Trial. JAMA Cardiol. 2017;2:547–55.
Lemos PA, Hoye A, Goedhart D, et al. Clinical, angiographic, and procedural predictors of angiographic restenosis after sirolimus-eluting stent implantation in complex patients: an evaluation from the Rapamycin-Eluting Stent Evaluated At Rotterdam Cardiology Hospital (RESEARCH) study. Circulation. 2004;109:1366–70.
Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation. 2019;139:e1082–143.
Giugliano RP, Keech A, Murphy SA, et al. Clinical efficacy and safety of evolocumab in high-risk patients receiving a statin: secondary analysis of patients with low LDL cholesterol levels and in those already receiving a maximal-potency statin in a randomized clinical trial. JAMA Cardiol. 2017;2:1385–91.
Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–97.
Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA 2005; 294:2437–45.
Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine (SEARCH) Collaborative Group, Armitage J, Bowman L. Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial. Lancet 2010; 376:1658–69.
Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005; 366: 1267–78.
Sabatine MS. PCSK9 inhibitors: what we know, what we should have understood, and what is to come. Eur Heart J. 2019. https://doi.org/10.1093/eurheartj/ehz514 [Epub ahead of print].
Guedeney P, Giustino G, Sorrentino S, et al. Efficacy and safety of alirocumab and evolocumab: a systematic review and meta-analysis of randomized controlled trials. Eur Heart J 2019. https://doi.org/10.1093/eurheartj/ehz430 [Epub ahead of print].
Ridker PM, Revkin J, Amarenco P, et al. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med 2017; 376:1527–39.
Steg PG, Szarek M, Bhatt DL, et al. Effect of alirocumab on mortality after acute coronary syndromes: an analysis of the ODYSSEY OUTCOMES randomized clinical trial. Circulation. 2019;140:103–12.
Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144–53.
Schmidt AF, Swerdlow DI, Holmes MV, et al. PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol 2017;5:97–105.
Mbikay M, Sirois F, Mayne J, et al. PCSK9-deficient mice exhibit impaired glucose tolerance and pancreatic islet abnormalities. FEBS Lett. 2010;584:701–6.
Sabatine MS, Giugliano RP, Wiviott SD, et al. Open-label study of long-term evaluation against LDL cholesterol (OSLER) investigators efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–9.
Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med 2015; 372:1489–99.
Giugliano RP, Mach F, Zavitz K, et al.Cognitive function in a randomized trial of evolocumab. N Engl J Med 2017; 377:633–643.
Fonarow GC, van Hout B, Villa G, Arellano J, Lindgren P. Updated cost-effectiveness analysis of evolocumab in patients with very high-risk atherosclerotic cardiovascular disease. JAMA Cardiol. 2019;4:691–5.
Nishikido T, Ray KK. Non-antibody approaches to proprotein convertase subtilisin kexin 9 inhibition: siRNA, antisense oligonucleotides, adnectins, vaccination, and new attempts at small-molecule inhibitors based on new discoveries. Front Cardiovasc Med. 2019;5:199.
Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373–84.
Koren MJ, Sabatine MS, Giugliano RP, et al. Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J Am Coll Cardiol. 2019;74:2132–46.
Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–13.
Acknowledgements
Funding
Remo H.M. Furtado was supported by the Lemann Foundation Cardiovascular Research Postdoctoral Fellowship—Harvard University/Brigham and Women´s Hospital. No other funding or sponsorship was received for this study. No Rapid Service Fee was received by the journal for the publication of this article.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Remo H.M. Furtado: Honoraria, modest; AstraZeneca, research grant, modest; AstraZeneca, DalCor, Boehinger, Pfizer, Bayer, Sanofi. Robert P. Giugliano: Research grant, significant; Amgen, Merck, honoraria, significant; Amgen, Daiichi Sankyo, Merck, consultant/advisory board, significant; Amarin, Amgen, Boehringer-Ingelheim, Bristol-Myers-Squibb, CVS Caremark, Daiichi Sankyo, GlaxoSmithKline, Lexicon, Merck, Portola, Pfizer. Robert P. Giugliano is also is an Editor-in-Chief of Cardiology and Therapy.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
Enhanced Digital Features
To view enhanced digital features for this article go to https://doi.org/10.6084/m9.figshare.11619465.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any non-commercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Furtado, R.H.M., Giugliano, R.P. What Lessons Have We Learned and What Remains to be Clarified for PCSK9 Inhibitors? A Review of FOURIER and ODYSSEY Outcomes Trials. Cardiol Ther 9, 59–73 (2020). https://doi.org/10.1007/s40119-020-00163-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40119-020-00163-w