Abstract
Background/Objectives:
Diet-induced weight loss is accompanied by compensatory changes, which increase appetite and encourage weight regain. There is some evidence that ketogenic diets suppress appetite. The objective is to examine the effect of ketosis on a number of circulating factors involved in appetite regulation, following diet-induced weight loss.
Subjects/Methods:
Of 50 non-diabetic overweight or obese subjects who began the study, 39 completed an 8-week ketogenic very-low-energy diet (VLED), followed by 2 weeks of reintroduction of foods. Following weight loss, circulating concentrations of glucose, insulin, non-esterified fatty acids (NEFA), β-hydroxybutyrate (BHB), leptin, gastrointestinal hormones and subjective ratings of appetite were compared when subjects were ketotic, and after refeeding.
Results:
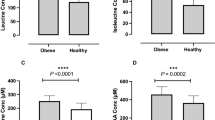
During the ketogenic VLED, subjects lost 13% of initial weight and fasting BHB increased from (mean±s.e.m.) 0.07±0.00 to 0.48±0.07 mmol/l (P<0.001). BHB fell to 0.19±0.03 mmol/l after 2 weeks of refeeding (P<0.001 compared with week 8). When participants were ketotic, the weight loss induced increase in ghrelin was suppressed. Glucose and NEFA were higher, and amylin, leptin and subjective ratings of appetite were lower at week 8 than after refeeding.
Conclusions:
The circulating concentrations of several hormones and nutrients which influence appetite were altered after weight loss induced by a ketogenic diet, compared with after refeeding. The increase in circulating ghrelin and subjective appetite which accompany dietary weight reduction were mitigated when weight-reduced participants were ketotic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Nordmann AJ, Nordmann A, Briel M, Keller U, Yancy WS Jr., Brehm BJ et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch Intern Med 2006; 166: 285–293.
Astrup A, Meinert Larsen T, Harper A . Atkins and other low-carbohydrate diets: hoax or an effective tool for weight loss? Lancet 2004; 364: 897–899.
Erlanson-Albertsson C, Mei J . The effect of low carbohydrate on energy metabolism. Int J Obes (Lond) 2005; 29 (Suppl 2), S26–S30.
Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP . Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann Intern Med 2005; 142: 403–411.
Yancy WS Jr., Olsen MK, Guyton JR, Bakst RP, Westman EC . A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Ann Intern Med 2004; 140: 769–777.
Schwartz MW, Woods SC, Porte D Jr., Seeley RJ, Baskin DG . Central nervous system control of food intake. Nature 2000; 404: 661–671.
Campfield LA, Smith FJ, Rosenbaum M, Hirsch J . Human eating: evidence for a physiological basis using a modified paradigm. Neurosci Biobehav Rev 1996; 20: 133–137.
Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L . Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002; 51: 271–275.
Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL et al. Gut hormone PYY[sub3-36] physiologically inhibits food intake. Nature 2002; 418: 650–654.
Batterham RL, Le Roux CW, Cohen MA, Park AJ, Ellis SM, Patterson M et al. Pancreatic polypeptide reduces appetite and food intake in humans. J Clin Endocrinol Metab 2003; 88: 3989–3992.
Gutzwiller JP, Goke B, Drewe J, Hildebrand P, Ketterer S, Handschin D et al. Glucagon-like peptide-1: a potent regulator of food intake in humans. Gut 1999; 44: 81–86.
Moran TH, Kinzig KP . Gastrointestinal satiety signals II. Cholecystokinin. Am J Physiol Gastrointest Liver Physiol 2004; 286: G183–G188.
Rezek M . The role of insulin in the glucostatic control of food intake. Can J Physiol Pharmacol 1976; 54: 650–665.
Wren AM, Seal LJ, Cohen MA, Brynes AE, Frost GS, Murphy KG et al. Ghrelin enhances appetite and increases food intake in humans. J Clin Endocrinol Metab 2001; 86: 5992–5995.
Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM . Positional cloning of the mouse obese gene and its human homologue. Nature 1994; 372: 425–432.
Leibel RL, Hirsch J . Diminished energy requirements in reduced-obese patients. Metabolism 1984; 33: 164–170.
Geldszus R, Mayr B, Horn R, Geisthovel F, von zur Muhlen A, Brabant G . Serum leptin and weight reduction in female obesity. Eur J Endocrinol 1996; 135: 659–662.
Cummings DE, Weigle DS, Frayo RS, Breen PA, Ma MK, Dellinger EP et al. Plasma ghrelin levels after diet-induced weight loss or gastric bypass surgery. N Engl J Med 2002; 346: 1623–1630.
Chearskul S, Delbridge E, Shulkes A, Proietto J, Kriketos A . Effect of weight loss and ketosis on postprandial cholecystokinin and free fatty acid concentrations. Am J Clin Nutr 2008; 87: 1238–1246.
Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A et al. Long-term persistence of hormonal adaptations to weight loss. New Engl J Med 2011; 365: 1597–1604.
Stubbs RJ, Hughes DA, Johnstone AM, Rowley E, Reid C, Elia M et al. The use of visual analogue scales to assess motivation to eat in human subjects: a review of their reliability and validity with an evaluation of new hand-held computerized systems for temporal tracking of appetite ratings. Br J Nutr 2000; 84: 405–415.
Hall SE, Wastney ME, Bolton TM, Braaten JT, Berman M . Ketone body kinetics in humans: the effects of insulin-dependent diabetes, obesity and starvation. J Lipid Res 1984; 25: 1184–1194.
R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing: Vienna, 2011.
Benjamini Y, Yekutieli D . The control of the false discovery rate in multiple testing under dependency. Ann Statist 2001; 29: 1165–1188.
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC . Homoeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419.
Pfluger PT, Kampe J, Castaneda TR, Vahl T, D'Alessio DA, Kruthaupt T et al. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J Clin Endocrinol Metab 2007; 92: 583–588.
Ratliff J, Mutungi G, Puglisi MJ, Volek JS, Fernandez ML . Carbohydrate restriction (with or without additional dietary cholesterol provided by eggs) reduces insulin resistance and plasma leptin without modifying appetite hormones in adult men. Nutr Res 2009; 29: 262–268.
Gil KM, Skeie B, Kvetan V, Askanazi J, Friedman MI . Parenteral nutrition and oral intake: effect of glucose and fat infusions. J Parenter Enteral Nutr 1991; 15: 426–432.
Mayer J . Glucostatic mechanism of regulation of food intake. N Engl J Med 1953; 249: 13–16.
Samaha FF, Iqbal N, Seshadri P, Chicano KL, Daily DA, McGrory J et al. A low-carbohydrate as compared with a low-fat diet in severe obesity. N Engl J Med 2003; 348: 2074–2081.
Volek JS, Sharman MJ, Gomez AL, DiPasquale C, Roti M, Pumerantz A et al. Comparison of a very low-carbohydrate and low-fat diet on fasting lipids, LDL subclasses, insulin resistance, and postprandial lipemic responses in overweight women. J Am Coll Nutr 2004; 23: 177–184.
Bisschop PH, de Metz J, Ackermans MT, Endert E, Pijl H, Kuipers F et al. Dietary fat content alters insulin-mediated glucose metabolism in healthy men. Am J Clin Nutr 2001; 73: 554–559.
Liu J, Jahn LA, Fowler DE, Barrett EJ, Cao W, Liu Z . Free fatty acids induce insulin resistance in both cardiac and skeletal muscle microvasculature in humans. J Clin Endocrinol Metab 2011; 96: 438–446.
Arase K, Fisler JS, Shargill NS, York DA, Bray GA . Intracerebroventricular infusions of 3-OHB and insulin in a rat model of dietary obesity. Am J Physiol 1988; 255: R974–R981.
Laeger T, Pohland R, Metges CC, Kuhla B . The ketone body beta-hydroxybutyric acid influences agouti-related peptide expression via AMP-activated protein kinase in hypothalamic GT1-7 cells. J Endocrinol 2012; 213: 193–203.
Langhans W, Egli G, Scharrer E . Selective hepatic vagotomy eliminates the hypophagic effect of different metabolites. J Auton Nerv Syst 1985; 13: 255–262.
Horn CC, Friedman MI . Separation of hepatic and gastrointestinal signals from the common ‘hepatic’ branch of the vagus. Am J Physiol Regul Integr Comp Physiol 2004; 287: R120–R126.
Date Y, Murakami N, Toshinai K, Matsukura S, Niijima A, Matsuo H et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002; 123: 1120–1128.
Rosenbaum M, Nicolson M, Hirsch J, Murphy E, Chu F, Leibel RL . Effects of weight change on plasma leptin concentrations and energy expenditure. J Clin Endocrinol Metab 1997; 82: 3647–3654.
Riediger T, Schmid HA, Lutz T, Simon E . Amylin potently activates AP neurons possibly via formation of the excitatory second messenger cGMP. Am J Physiol Regul Integr Comp Physiol 2001; 281: R1833–R1843.
Lutz TA, Tschudy S, Rushing PA, Scharrer E . Attenuation of the anorectic effects of cholecystokinin and bombesin by the specific amylin antagonist AC 253. Physiol Behav 2000; 70: 533–536.
Hauner H, Glatting G, Kaminska D, Pfeiffer EF . Effects of gastric inhibitory polypeptide on glucose and lipid metabolism of isolated rat adipocytes. Ann Nutr Metab 1988; 32: 282–288.
Foster GD, Wyatt HR, Hill JO, McGuckin BG, Brill C, Mohammed BS et al. A randomized trial of a low-carbohydrate diet for obesity. N Engl J Med 2003; 348: 2082–2090.
Garcia JM, Iyer D, Poston WSC, Marcelli M, Reeves R, Foreyt J et al. Rise of plasma ghrelin with weight loss is not sustained during weight maintenance. Obesity (Silver Spring) 2006; 14: 1716–1723.
Visinoni S, Khalid NFI, Joannides CN, Shulkes A, Yim M, Whitehead J et al. The role of liver fructose-1,6-bisphosphatase in regulating appetite and adiposity. Diabetes 2012; 61: 1122–1132.
Acknowledgements
This work was supported by NHMRC project grant (508920), Endocrine Society of Australia scholarship (PS), Royal Australasian College of Physicians Shields Research Entry scholarship (PS), and Sir Edward Dunlop Medical Research Foundation (JP). We thank Celestine Bouniu, John Cardinal, Sherrell Cardinal, Christian Rantzau, Rebecca Sgambellone, Sherley Visinoni and Mildred Yim for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
JP was chairman of the Optifast medical advisory board at the time the study was conducted. The other authors have no conflict of interest.
Additional information
Supplementary Information accompanies this paper on European Journal of Clinical Nutrition website
Supplementary information
Rights and permissions
About this article
Cite this article
Sumithran, P., Prendergast, L., Delbridge, E. et al. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur J Clin Nutr 67, 759–764 (2013). https://doi.org/10.1038/ejcn.2013.90
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ejcn.2013.90
Keywords
This article is cited by
-
Effectiveness of a telehealth-delivered clinician-supported exercise and weight loss program for hip osteoarthritis – protocol for the Better Hip randomised controlled trial
BMC Musculoskeletal Disorders (2024)
-
A 6-Week Ketogenic Diet Enhances the Phosphocreatine Energy System Contribution During Intermittent Sprints
Journal of Science in Sport and Exercise (2024)
-
Metabolic shift toward ketosis in asocial cavefish increases social-like affinity
BMC Biology (2023)
-
Effectiveness of a telehealth physiotherapist-delivered intensive dietary weight loss program combined with exercise in people with knee osteoarthritis and overweight or obesity: study protocol for the POWER randomized controlled trial
BMC Musculoskeletal Disorders (2022)
-
Ketogenic diet for human diseases: the underlying mechanisms and potential for clinical implementations
Signal Transduction and _targeted Therapy (2022)