Abstract
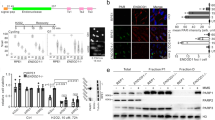
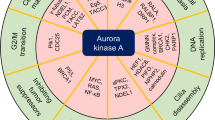
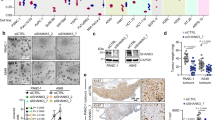
The proto-oncogene KRAS is mutated in a wide array of human cancers, most of which are aggressive and respond poorly to standard therapies. Although the identification of specific oncogenes has led to the development of clinically effective, molecularly _targeted therapies in some cases, KRAS has remained refractory to this approach. A complementary strategy for _targeting KRAS is to identify gene products that, when inhibited, result in cell death only in the presence of an oncogenic allele1,2. Here we have used systematic RNA interference to detect synthetic lethal partners of oncogenic KRAS and found that the non-canonical IκB kinase TBK1 was selectively essential in cells that contain mutant KRAS. Suppression of TBK1 induced apoptosis specifically in human cancer cell lines that depend on oncogenic KRAS expression. In these cells, TBK1 activated NF-κB anti-apoptotic signals involving c-Rel and BCL-XL (also known as BCL2L1) that were essential for survival, providing mechanistic insights into this synthetic lethal interaction. These observations indicate that TBK1 and NF-κB signalling are essential in KRAS mutant tumours, and establish a general approach for the rational identification of co-dependent pathways in cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Hartwell, L. H., Szankasi, P., Roberts, C. J., Murray, A. W. & Friend, S. H. Integrating genetic approaches into the discovery of anticancer drugs. Science 278, 1064–1068 (1997)
Kaelin, W. G. The concept of synthetic lethality in the context of anticancer therapy. Nature Rev. Cancer 5, 689–698 (2005)
Moffat, J. et al. A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124, 1283–1298 (2006)
Malo, N., Hanley, J. A., Cerquozzi, S., Pelletier, J. & Nadon, R. Statistical practice in high-throughput screening data analysis. Nature Biotechnol. 24, 167–175 (2006)
Gould, J., Getz, G., Monti, S., Reich, M. & Mesirov, J. P. Comparative gene marker selection suite. Bioinformatics 22, 1924–1925 (2006)
Luo, B. et al. Highly parallel identification of essential genes in cancer cells. Proc. Natl Acad. Sci. USA 105, 20380–20385 (2008)
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005)
Lundberg, A. S. et al. Immortalization and transformation of primary human airway epithelial cells by gene transfer. Oncogene 21, 4577–4586 (2002)
Wislez, M. et al. Inhibition of mammalian _target of rapamycin reverses alveolar epithelial neoplasia induced by oncogenic K-ras. Cancer Res. 65, 3226–3235 (2005)
Chien, Y. et al. RalB GTPase-mediated activation of the IκB family kinase TBK1 couples innate immune signaling to tumor cell survival. Cell 127, 157–170 (2006)
Häcker, H. & Karin, M. Regulation and function of IKK and IKK-related kinases. Sci. STKE 2006, re13 (2006)
Bild, A. H. et al. Oncogenic pathway signatures in human cancers as a guide to _targeted therapies. Nature 439, 353–357 (2006)
Hinata, K., Gervin, A. M., Jennifer Zhang, Y. & Khavari, P. A. Divergent gene regulation and growth effects by NF-κB in epithelial and mesenchymal cells of human skin. Oncogene 22, 1955–1964 (2003)
Boehm, J. S. et al. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell 129, 1065–1079 (2007)
Ding, L. et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 455, 1069–1075 (2008)
Takeuchi, T. et al. Expression profile-defined classification of lung adenocarcinoma shows close relationship with underlying major genetic changes and clinicopathologic behaviors. J. Clin. Oncol. 24, 1679–1688 (2006)
Andersen, J., VanScoy, S., Cheng, T. F., Gomez, D. & Reich, N. C. IRF-3-dependent and augmented _target genes during viral infection. Genes Immun. 9, 168–175 (2008)
Singh, A. et al. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell 15, 489–500 (2009)
Mayo, M. W. et al. Requirement of NF-κB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science 278, 1812–1815 (1997)
Brown, K., Gerstberger, S., Carlson, L., Franzoso, G. & Siebenlist, U. Control of IκB-α proteolysis by site-specific, signal-induced phosphorylation. Science 267, 1485–1488 (1995)
Harris, J. et al. Nuclear accumulation of cRel following C-terminal phosphorylation by TBK1/IKKε. J. Immunol. 177, 2527–2535 (2006)
Mercurio, F., DiDonato, J. A., Rosette, C. & Karin, M. p105 and p98 precursor proteins play an active role in NF-κB-mediated signal transduction. Genes Dev. 7, 705–718 (1993)
Owyang, A. M. et al. c-Rel is required for the protection of B cells from antigen receptor-mediated, but not Fas-mediated, apoptosis. J. Immunol. 167, 4948–4956 (2001)
Scholl, C. et al. Synthetic lethal interaction between oncogenic KRAS dependency and STK33 suppression in human cancer cells. Cell 137, 821–834 (2009)
Luo, J. et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell 137, 835–848 (2009)
Ngo, V. N. et al. A loss-of-function RNA interference screen for molecular _targets in cancer. Nature 441, 106–110 (2006)
Rottmann, S., Wang, Y., Nasoff, M., Deveraux, Q. L. & Quon, K. C. A. TRAIL receptor-dependent synthetic lethal relationship between MYC activation and GSK3β/FBW7 loss of function. Proc. Natl Acad. Sci. USA 102, 15195–15200 (2005)
Silva, J. M. et al. Profiling essential genes in human mammary cells by multiplex RNAi screening. Science 319, 617–620 (2008)
Zhang, J. Powerful goodness-of-fit tests based on the likelihood ratio. J. R. Stat. Soc. Series B Stat. Methodol. 64, 281–294 (2002)
Sos, M. L. et al. Predicting drug susceptibility of non-small cell lung cancers based on genetic lesions. J. Clin. Invest. 119, 1727–1740 (2009)
Bhattacharjee, A. et al. Classification of human lung carcinomas by mRNA expression profiling reveals distinct adenocarcinoma subclasses. Proc. Natl Acad. Sci. USA 98, 13790–13795 (2001)
Acknowledgements
This work was supported in part by grants from the US National Cancer Institute (R33 CA128625, R01 CA130988) (W.C.H.) and NIH T32 CA09172-33 (D.A.B., S.E.M.), the Starr Cancer Consortium (I1-A11; W.C.H., D.G.G.), the Susan Madden Fund and an ASCO YIA (D.A.B.), a Department of Defense Prostate Cancer Postdoctoral Fellowship (S.Y.K.), a Brain Science Foundation Fellowship (I.F.D.), the Deutsche Krebshilfe (grant 107954) (R.K.T.), the Fritz-Thyssen-Stiftung (grant 10.08.2.175; R.K.T.) and the NGFNplus-program of the German Ministry of Science and Education (BMBF, grant 01GS08100; R.K.T.). We thank C. Yu, G. Wei and members of the Hahn laboratory for discussions. High-throughput RNAi screening was conducted at the RNAi Platform of the Broad Institute of MIT and Harvard.
Author Contributions D.A.B., J.S.B., S.Y.K., S.E.M. and W.C.H. designed the experiments. D.A.B. and P.T. performed computational analyses. S.Y.K., I.F.D., A.C.S., P.S., C.S., S.F., P.B.G., J.H.R., Q.S. and R.C.W. performed primary RNAi screens; S.J.S., S.H., B.S.W., C.M. and B.A.W. assisted with data analysis. D.A.B. performed secondary screen with help from H.L. S.E.M. performed tumour xenograft experiments. E.M. performed experiments with murine cell lines. D.A.B., J.S.B., E.M.C., M.L.S., K.M. and R.K.T. performed expression-profiling experiments. S.R., D.M.L., D.M.S., E.S.L., D.G.G., T.J. and D.E.R. supervised RNAi screens; M.M. and J.P.M. supervised data analysis. D.A.B. and W.C.H. wrote the manuscript. W.C.H. coordinated all aspects of the project. All authors discussed results and edited the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
W.C.H., M.M. and D.M.L. are consultants for Novartis Pharmaceuticals, Inc.
Supplementary information
Supplementary Information
This file contains Supplementary Figures 1-11 with Legends and Supplementary Tables 1-5. (PDF 3910 kb)
Rights and permissions
About this article
Cite this article
Barbie, D., Tamayo, P., Boehm, J. et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature 462, 108–112 (2009). https://doi.org/10.1038/nature08460
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nature08460
This article is cited by
-
Individualized detection of TMPRSS2-ERG fusion status in prostate cancer: a rank-based qualitative transcriptome signature
World Journal of Surgical Oncology (2024)
-
ITGAM-mediated macrophages contribute to basement membrane damage in diabetic nephropathy and atherosclerosis
BMC Nephrology (2024)
-
A gene signature linked to fibroblast differentiation for prognostic prediction of mesothelioma
Cell & Bioscience (2024)
-
Immune-related lncRNAs signature and radiomics signature predict the prognosis and immune microenvironment of glioblastoma multiforme
Journal of Translational Medicine (2024)
-
Development and validation of a disulfidptosis and disulfide metabolism-related risk index for predicting prognosis in lung adenocarcinoma
Cancer Cell International (2024)