Key Points
-
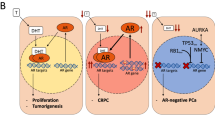
Prostate cancer pathogenesis is dependent on signalling through the steroid nuclear hormone androgen receptor (AR), which is activated after binding of the androgen ligand testosterone or dihydrotestosterone. Ligand-bound AR translocates to the nucleus, where it serves to induce or repress gene expression through binding to chromatin at cis androgen response elements.
-
Medical castration to substantially deplete serum testosterone is the mainstay of therapy for advanced prostate cancer that recurs following surgical removal of the prostate (prostatectomy) or radiotherapy. However, castration therapy is not curative, and patients will eventually progress to lethal castration-resistant prostate cancer (CRPC).
-
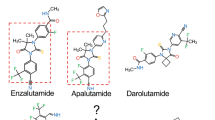
Despite a castrate level of testosterone, CRPC almost uniformly remains dependent on AR signalling. Next-generation hormonal therapies for prostate cancer, abiraterone and enzalutamide, are now in widespread clinical use; abiraterone attacks AR signalling through inhibition of extra-gonadal androgen biosynthesis and enzalutamide interferes directly with androgen binding to AR.
-
Resistance mechanisms to these drugs have been identified that result in restoration of AR signalling through gain-of-function AR mutations, upregulation of constitutively active AR splice variants or increased intratumoural androgen biosynthesis. Another resistance mechanism bypasses AR by switching to the related glucocorticoid receptor (GR) to maintain transcriptional regulation of a subset of the same genes.
-
At resistance, a subset of patients are now presenting with low or no AR in their tumours, suggesting that evolution to complex genomic states completely independently of AR could increasingly become a cause for concern.
-
Comprehensive analyses of late-stage CRPC are uncovering multiple genetic lesions in this patient cohort that indicate that it may eventually be possible to stratify patients based on the genomic profile of their cancer. These efforts will aid in clinical trial design and facilitate the use of rationally designed combination strategies to improve patient outcomes.
Abstract
During the past 10 years, preclinical studies implicating sustained androgen receptor (AR) signalling as the primary driver of castration-resistant prostate cancer (CRPC) have led to the development of novel agents _targeting the AR pathway that are now in widespread clinical use. These drugs prolong the survival of patients with late-stage prostate cancer but are not curative. In this Review, we highlight emerging mechanisms of acquired resistance to these contemporary therapies, which fall into the three broad categories of restored AR signalling, AR bypass signalling and complete AR independence. This diverse range of resistance mechanisms presents new challenges for long-term disease control, which may be addressable through early use of combination therapies guided by recent insights from genomic landscape studies of CRPC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Haas, G. P., Delongchamps, N., Brawley, O. W., Wang, C. Y. & de la Roza, G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can. J. Urol. 15, 3866–3871 (2008).
National Cancer Institute. SEER Stat Fact Sheets: Prostate Cancer National Cancer Institute [online], (2012).
Buzzoni, C. et al. Metastatic prostate cancer incidence and prostate-specific antigen testing: new insights from the European Randomized Study of Screening for Prostate Cancer. Eur. Urol. 68, 885–890 (2015).
Huggins, C., Stevens, R. E. Jr & Hodges, C. V. Studies on prostatic cancer II. The effects of castration on advanced carcinoma of the prostate gland. Arch. Surg. 43, 209–223 (1941).
van Poppel, H. & Nilsson, S. Testosterone surge: rationale for gonadotropin-releasing hormone blockers? Urology 71, 1001–1006 (2008).
Visakorpi, T. et al. In vivo amplification of the androgen receptor gene and progression of human prostate cancer. Nat. Genet. 9, 401–406 (1995). This was the first paper to establish that AR undergoes frequent genomic amplification in prostate cancer during progression to castration resistance.
Chen, C. D. et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 (2004). Modest overexpression of AR in prostate cancer cells was sufficient to confer resistance to AR inhibition, in part by facilitating the conversion of an anti-androgen into a transcriptional agonist.
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Attard, G., Belldegrun, A. S. & de Bono, J. S. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 96, 1241–1246 (2005).
Scher, H. I. et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 367, 1187–1197 (2012). In a Phase III trial, enzalutamide prolonged the survival of patients with metastatic CRPC refractory to docetaxel by nearly 5 months.
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011). In a Phase III trial, abiraterone conferred a nearly 4-month survival advantage to patients with metastatic CRPC refractory to docetaxel.
Ryan, C. J. et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 16, 152–160 (2015).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Veldscholte, J. et al. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem. Biophys. Res. Commun. 173, 534–540 (1990).
Suzuki, H. et al. Androgen receptor gene mutations in human prostate cancer. J. Steroid Biochem. Mol. Biol. 46, 759–765 (1993).
Barbieri, C. E. et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 44, 685–689 (2012).
Beltran, H. et al. _targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic _targets and disease heterogeneity. Eur. Urol. 63, 920–926 (2013).
Grasso, C. S. et al. The mutational landscape of lethal castration-resistant prostate cancer. Nature 487, 239–243 (2012).
Taylor, B. S. et al. Integrative genomic profiling of human prostate cancer. Cancer Cell 18, 11–22 (2010).
Robinson, D. et al. Integrative clinical genomics of advanced prostate cancer. Cell 161, 1215–1228 (2015). A multi-institutional effort that generated whole-exome and transcriptome sequencing from 150 patients with metastatic CRPC. This study identified clinically actionable genomic alterations in 89% of the patients, highlighting possible avenues for personalized medicine.
Gottlieb, B., Beitel, L. K., Nadarajah, A., Paliouras, M. & Trifiro, M. The androgen receptor gene mutations database: 2012 update. Hum. Mutat. 33, 887–894 (2012).
Tan, J. et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol. Endocrinol. 11, 450–459 (1997).
Taplin, M. E. et al. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 59, 2511–2515 (1999).
Scher, H. I. & Kelly, W. K. Flutamide withdrawal syndrome: its impact on clinical trials in hormone-refractory prostate cancer. J. Clin. Oncol. 11, 1566–1572 (1993).
Suzuki, H. et al. Codon 877 mutation in the androgen receptor gene in advanced prostate cancer: relation to antiandrogen withdrawal syndrome. Prostate 29, 153–158 (1996).
Hara, T. et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 63, 149–153 (2003).
Balbas, M. D. et al. Overcoming mutation-based resistance to antiandrogens with rational drug design. eLife 2, e00499 (2013).
Clegg, N. J. et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 72, 1494–1503 (2012).
Joseph, J. D. et al. A clinically relevant androgen receptor mutation confers resistance to second-generation antiandrogens enzalutamide and ARN-509. Cancer Discov. 3, 1020–1029 (2013).
Azad, A. A. et al. Androgen receptor gene aberrations in circulating cell-free DNA: biomarkers of therapeutic resistance in castration-resistant prostate cancer. Clin. Cancer Res. 21, 2315–2324 (2015).
Zhao, X. Y. et al. Glucocorticoids can promote androgen-independent growth of prostate cancer cells through a mutated androgen receptor. Nat. Med. 6, 703–706 (2000).
van de Wijngaart, D. J. et al. Systematic structure-function analysis of androgen receptor Leu701 mutants explains the properties of the prostate cancer mutant L701H. J. Biol. Chem. 285, 5097–5105 (2010).
Chen, E. J. et al. Abiraterone treatment in castration-resistant prostate cancer selects for progesterone responsive mutant androgen receptors. Clin. Cancer Res. 21, 1273–1280 (2015).
Carreira, S. et al. Tumor clone dynamics in lethal prostate cancer. Sci. Transl Med. 6, 254ra125 (2014).
Attard, G. et al. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J. Clin. Endocrinol. Metab. 97, 507–516 (2012).
Ware, K. E., Garcia-Blanco, M. A., Armstrong, A. J. & Dehm, S. M. Biologic and clinical significance of androgen receptor variants in castration resistant prostate cancer. Endocr. Relat. Cancer 21, T87–T103 (2014).
Nakazawa, M., Antonarakis, E. S. & Luo, J. Androgen receptor splice variants in the era of enzalutamide and abiraterone. Horm. Cancer 5, 265–273 (2014).
Dehm, S. M., Schmidt, L. J., Heemers, H. V., Vessella, R. L. & Tindall, D. J. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 68, 5469–5477 (2008).
Watson, P. A. et al. Constitutively active androgen receptor splice variants expressed in castration-resistant prostate cancer require full-length androgen receptor. Proc. Natl Acad. Sci. USA 107, 16759–16765 (2010). Overexpression of ARVs in which the LBD was deleted was not sufficient to confer growth resistance to enzalutamide, suggesting that in certain cellular contexts the ARVs are not fully capable of recapitulating the function of the full-length receptor.
Guo, Z. et al. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 69, 2305–2313 (2009).
Hu, R. et al. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 69, 16–22 (2009).
Sun, S. et al. Castration resistance in human prostate cancer is conferred by a frequently occurring androgen receptor splice variant. J. Clin. Invest. 120, 2715–2730 (2010).
Hu, R., Isaacs, W. B. & Luo, J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate 71, 1656–1667 (2011).
Jenster, G., Trapman, J. & Brinkmann, A. O. Nuclear import of the human androgen receptor. Biochem. J. 293, 761–768 (1993).
Hornberg, E. et al. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS ONE 6, e19059 (2011).
Quarmby, V. E., Yarbrough, W. G., Lubahn, D. B., French, F. S. & Wilson, E. M. Autologous down-regulation of androgen receptor messenger ribonucleic acid. Mol. Endocrinol. 4, 22–28 (1990).
Shan, L. X., Rodriguez, M. C. & Janne, O. A. Regulation of androgen receptor protein and mRNA concentrations by androgens in rat ventral prostate and seminal vesicles and in human hepatoma cells. Mol. Endocrinol. 4, 1636–1646 (1990).
Yu, Z. et al. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin. Cancer Res. 20, 1590–1600 (2014).
Cai, C. et al. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell 20, 457–471 (2011).
Liu, L. L. et al. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene 33, 3140–3150 (2014).
Li, Y. et al. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 73, 483–489 (2013). In prostate cancer cells with naturally high expression of LBD-truncated ARVs and inherent anti-androgen resistance, variant-specific knockdown conferred sensitivity to enzalutamide.
Li, Y. et al. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res. 71, 2108–2117 (2011).
Li, Y. et al. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene 31, 4759–4767 (2012).
Sun, F. et al. Androgen receptor splice variant AR3 promotes prostate cancer via modulating expression of autocrine/paracrine factors. J. Biol. Chem. 289, 1529–1539 (2014).
Liu, G. et al. AR variant ARv567es induces carcinogenesis in a novel transgenic mouse model of prostate cancer. Neoplasia 15, 1009–1017 (2013).
Efstathiou, E. et al. Molecular characterization of enzalutamide-treated bone metastatic castration-resistant prostate cancer. Eur. Urol. 67, 53–60 (2015).
Antonarakis, E. S. et al. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N. Engl. J. Med. 371, 1028–1038 (2014).
Bramson, H. N. et al. Unique preclinical characteristics of GG745, a potent dual inhibitor of 5AR. J. Pharmacol. Exp. Ther. 282, 1496–1502 (1997).
Steers, W. D. 5α reductase activity in the prostate. Urology 58 (6 Suppl. 1), 17–24.
Nishiyama, T., Hashimoto, Y. & Takahashi, K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin. Cancer Res. 10, 7121–7126 (2004).
Titus, M. A., Schell, M. J., Lih, F. B., Tomer, K. B. & Mohler, J. L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 11, 4653–4657 (2005).
Montgomery, R. B. et al. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 68, 4447–4454 (2008).
Page, S. T. et al. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J. Clin. Endocrinol. Metab. 91, 3850–3856 (2006).
Tamae, D. et al. The DHEA-sulfate depot following P450c17 inhibition supports the case for AKR1C3 inhibition in high risk localized and advanced castration resistant prostate cancer. Chem. Biol. Interact. 234, 332–338 (2015).
Dufort, I., Rheault, P., Huang, X. F., Soucy, P. & Luu-The, V. Characteristics of a highly labile human type 5 17β-hydroxysteroid dehydrogenase. Endocrinology 140, 568–574 (1999).
Koh, E., Noda, T., Kanaya, J. & Namiki, M. Differential expression of 17β-hydroxysteroid dehydrogenase isozyme genes in prostate cancer and noncancer tissues. Prostate 53, 154–159 (2002).
Chang, K. H. et al. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl Acad. Sci. USA 108, 13728–13733 (2011).
Liu, C. et al. Intracrine androgens and AKR1C3 activation confer resistance to enzalutamide in prostate cancer. Cancer Res. 75, 1413–1422 (2015).
Cai, C. et al. Intratumoral de novo steroid synthesis activates androgen receptor in castration-resistant prostate cancer and is upregulated by treatment with CYP17A1 inhibitors. Cancer Res. 71, 6503–6513 (2011).
Adeniji, A. O., Chen, M. & Penning, T. M. AKR1C3 as a _target in castrate resistant prostate cancer. J. Steroid Biochem. Mol. Biol. 137, 136–149 (2013).
Chang, K. H. et al. A gain-of-function mutation in DHT synthesis in castration-resistant prostate cancer. Cell 154, 1074–1084 (2013).
Sawyers, C. L. in DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology (eds DeVita, V. T. D. Jr, Lawrence, T. S. & Rosenberg, S. A.) 237–247 (Wolters Kluwer Health, 2015).
Isikbay, M. et al. Glucocorticoid receptor activity contributes to resistance to androgen-_targeted therapy in prostate cancer. Horm. Cancer 5, 72–89 (2014).
Arora, V. K. et al. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell 155, 1309–1322 (2013). By acquiring expression of the GR, prostate cancer cells were shown to evade the antiproliferative effects of enzalutamide or ARN-509 by utilizing this related steroid hormone receptor to cross-regulate a subset of AR-regulated _target genes.
Feldman, B. J. & Feldman, D. The development of androgen-independent prostate cancer. Nat. Rev. Cancer 1, 34–45 (2001).
Pienta, K. J. & Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 12, 1665–1671 (2006).
Sahu, B. et al. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 73, 1570–1580 (2013).
Efstathiou, E. et al. Biological heterogeneity in localized high-risk prostate cancer (LHRPC) from a study of neoadjuvant abiraterone acetate plus leuprolide acetate (LHRHa) versus LHRHa. J. Clin. Oncol. 33 (15 Suppl.), 5005 (2015).
Tannock, I. et al. Treatment of metastatic prostatic cancer with low-dose prednisone: evaluation of pain and quality of life as pragmatic indices of response. J. Clin. Oncol. 7, 590–597 (1989).
Ryan, C. J. et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N. Engl. J. Med. 368, 138–148 (2013).
Song, L. N., Coghlan, M. & Gelmann, E. P. Antiandrogen effects of mifepristone on coactivator and corepressor interactions with the androgen receptor. Mol. Endocrinol. 18, 70–85 (2004).
Taplin, M. E. et al. A phase II study of mifepristone (RU-486) in castration-resistant prostate cancer, with a correlative assessment of androgen-related hormones. BJU Int. 101, 1084–1089 (2008).
Lu, N. Z. et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol. Rev. 58, 782–797 (2006).
Grindstad, T. et al. High progesterone receptor expression in prostate cancer is associated with clinical failure. PLoS ONE 10, e0116691 (2015).
Bonkhoff, H., Fixemer, T., Hunsicker, I. & Remberger, K. Progesterone receptor expression in human prostate cancer: correlation with tumor progression. Prostate 48, 285–291 (2001).
Mobbs, B. G. & Liu, Y. Immunohistochemical localization of progesterone receptor in benign and malignant human prostate. Prostate 16, 245–251 (1990).
Yu, Y. et al. Expression and function of the progesterone receptor in human prostate stroma provide novel insights to cell proliferation control. J. Clin. Endocrinol. Metab. 98, 2887–2896 (2013).
Roudier, M. P. et al. Phenotypic heterogeneity of end-stage prostate carcinoma metastatic to bone. Hum. Pathol. 34, 646–653 (2003).
Shah, R. B. et al. Androgen-independent prostate cancer is a heterogeneous group of diseases: lessons from a rapid autopsy program. Cancer Res. 64, 9209–9216 (2004).
Gundem, G. et al. The evolutionary history of lethal metastatic prostate cancer. Nature 520, 353–357 (2015).
Hobisch, A. et al. Distant metastases from prostatic carcinoma express androgen receptor protein. Cancer Res. 55, 3068–3072 (1995).
Efstathiou, E. et al. Effects of abiraterone acetate on androgen signaling in castrate-resistant prostate cancer in bone. J. Clin. Oncol. 30, 637–643 (2012).
Crnalic, S. et al. Nuclear androgen receptor staining in bone metastases is related to a poor outcome in prostate cancer patients. Endocr. Relat. Cancer 17, 885–895 (2010).
Palmgren, J. S., Karavadia, S. S. & Wakefield, M. R. Unusual and underappreciated: small cell carcinoma of the prostate. Semin. Oncol. 34, 22–29 (2007).
Deorah, S., Rao, M. B., Raman, R., Gaitonde, K. & Donovan, J. F. Survival of patients with small cell carcinoma of the prostate during 1973-2003: a population-based study. BJU Int. 109, 824–830 (2012).
Epstein, J. I. et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am. J. Surg. Pathol. 38, 756–767 (2014).
Tan, H. L. et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin. Cancer Res. 20, 890–903 (2014).
Beltran, H. et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug _targets. Cancer Discov. 1, 487–495 (2011).
Zhou, Z. et al. Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer. Cancer Res. 66, 7889–7898 (2006).
Lotan, T. L. et al. ERG gene rearrangements are common in prostatic small cell carcinomas. Mod. Pathol. 24, 820–828 (2011).
Guo, C. C. et al. TMPRSS2-ERG gene fusion in small cell carcinoma of the prostate. Hum. Pathol. 42, 11–17 (2011).
Williamson, S. R. et al. ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: evidence supporting monoclonal origin. Mod. Pathol. 24, 1120–1127 (2011).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Yuan, T. C., Veeramani, S. & Lin, M. F. Neuroendocrine-like prostate cancer cells: neuroendocrine transdifferentiation of prostate adenocarcinoma cells. Endocr. Relat. Cancer 14, 531–547 (2007).
Small, E. J. et al. Characterization of neuroendocrine prostate cancer (NEPC) in patients with metastatic castration resistant prostate cancer (mCRPC) resistant to abiraterone (Abi) or enzalutamide (Enz): Preliminary results from the SU2C/PCF/AACR West Coast Prostate Cancer Dream Team (WCDT). J. Clin. Oncol. 33 (15 Suppl.), 5003 (2015).
Schrader, A. J. et al. Enzalutamide in castration-resistant prostate cancer patients progressing after docetaxel and abiraterone. Eur. Urol. 65, 30–36 (2014).
Noonan, K. L. et al. Clinical activity of abiraterone acetate in patients with metastatic castration-resistant prostate cancer progressing after enzalutamide. Ann. Oncol. 24, 1802–1807 (2013).
Badrising, S. et al. Clinical activity and tolerability of enzalutamide (MDV3100) in patients with metastatic, castration-resistant prostate cancer who progress after docetaxel and abiraterone treatment. Cancer 120, 968–975 (2014).
Zhang, T. et al. Exploring the clinical benefit of docetaxel or enzalutamide after disease progression during abiraterone acetate and prednisone treatment in men with metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 13, 392–399 (2015).
Bianchini, D. et al. Antitumour activity of enzalutamide (MDV3100) in patients with metastatic castration-resistant prostate cancer (CRPC) pre-treated with docetaxel and abiraterone. Eur. J. Cancer 50, 78–84 (2014).
Brasso, K. et al. Enzalutamide antitumour activity against metastatic castration-resistant prostate cancer previously treated with docetaxel and abiraterone: a multicentre analysis. Eur. Urol. 68, 317–324 (2015).
Li, Z. et al. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature 523, 347–351 (2015).
Andersen, R. J. et al. Regression of castrate-recurrent prostate cancer by a small-molecule inhibitor of the amino-terminus domain of the androgen receptor. Cancer Cell 17, 535–546 (2010).
Dalal, K. et al. Selectively _targeting the DNA-binding domain of the androgen receptor as a prospective therapy for prostate cancer. J. Biol. Chem. 289, 26417–26429 (2014).
Osborne, C. K., Wakeling, A. & Nicholson, R. I. Fulvestrant: an oestrogen receptor antagonist with a novel mechanism of action. Br. J. Cancer 90 (Suppl. 1), S2–S6 (2004).
Omlin, A. et al. AZD3514, an oral selective androgen receptor down-regulator in patients with castration-resistant prostate cancer – results of two parallel first-in-human phase I studies. Invest. New Drugs 33, 679–690 (2015).
Bitting, R. L. & Armstrong, A. J. _targeting the PI3K/Akt/mTOR pathway in castration-resistant prostate cancer. Endocr. Relat. Cancer 20, R83–R99 (2013).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
Schwartz, S. et al. Feedback suppression of PI3Kα signaling in PTEN-mutated tumors is relieved by selective inhibition of PI3Kβ. Cancer Cell 27, 109–122 (2015).
Farmer, H. et al. _targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 434, 917–921 (2005).
Bryant, H. E. et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 434, 913–917 (2005).
Kaufman, B. et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 33, 244–250 (2015).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Sandhu, S. K. et al. The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 14, 882–892 (2013).
Mateo, J. et al. DNA repair defects and antitumor activity with PARP inhibition: TOPARP, a phase II trial of olaparib in metastatic castration resistant prostate cancer. Cancer Res. 75, CT322 (2015).
Lohr, J. G. et al. Whole-exome sequencing of circulating tumor cells provides a window into metastatic prostate cancer. Nat. Biotechnol. 32, 479–484 (2014).
Gao, D. et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 159, 176–187 (2014).
Sramkoski, R. M. et al. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell. Dev. Biol. Anim. 35, 403–409 (1999).
Pretlow, T. G. et al. Xenografts of primary human prostatic carcinoma. J. Natl Cancer Inst. 85, 394–398 (1993).
Dagvadorj, A. et al. Androgen-regulated and highly tumorigenic human prostate cancer cell line established from a transplantable primary CWR22 tumor. Clin. Cancer Res. 14, 6062–6072 (2008).
Stone, K. R., Mickey, D. D., Wunderli, H., Mickey, G. H. & Paulson, D. F. Isolation of a human prostate carcinoma cell line (DU 145). Int. J. Cancer 21, 274–281 (1978).
Mitchell, S., Abel, P., Ware, M., Stamp, G. & Lalani, E. Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU Int. 85, 932–944 (2000).
Klein, K. A. et al. Progression of metastatic human prostate cancer to androgen independence in immunodeficient SCID mice. Nat. Med. 3, 402–408 (1997).
Horoszewicz, J. S. et al. The LNCaP cell line—a new model for studies on human prostatic carcinoma. Prog. Clin. Biol. Res. 37, 115–132 (1980).
Horoszewicz, J. S. et al. LNCaP model of human prostatic carcinoma. Cancer Res. 43, 1809–1818 (1983).
Wu, H. C. et al. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int. J. Cancer 57, 406–412 (1994).
Navone, N. M. et al. Establishment of two human prostate cancer cell lines derived from a single bone metastasis. Clin. Cancer Res. 3, 2493–2500 (1997).
Zhao, X. Y. et al. Two mutations identified in the androgen receptor of the new human prostate cancer cell line MDA PCa 2a. J. Urol. 162, 2192–2199 (1999).
Kaighn, M. E., Narayan, K. S., Ohnuki, Y., Lechner, J. F. & Jones, L. W. Establishment and characterization of a human prostatic carcinoma cell line (PC-3). Invest. Urol. 17, 16–23 (1979).
Korenchuk, S. et al. VCaP, a cell-based model system of human prostate cancer. In Vivo 15, 163–168 (2001).
Liu, W. et al. Homozygous deletions and recurrent amplifications implicate new genes involved in prostate cancer. Neoplasia 10, 897–907 (2008).
Georget, V. et al. Trafficking of the androgen receptor in living cells with fused green fluorescent protein-androgen receptor. Mol. Cell. Endocrinol. 129, 17–26 (1997).
Georget, V., Terouanne, B., Nicolas, J. C. & Sultan, C. Mechanism of antiandrogen action: key role of hsp90 in conformational change and transcriptional activity of the androgen receptor. Biochemistry 41, 11824–11831 (2002).
Wong, C. I., Zhou, Z. X., Sar, M. & Wilson, E. M. Steroid requirement for androgen receptor dimerization and DNA binding. Modulation by intramolecular interactions between the NH2-terminal and steroid-binding domains. J. Biol. Chem. 268, 19004–19012 (1993).
Shang, Y., Myers, M. & Brown, M. Formation of the androgen receptor transcription complex. Mol. Cell 9, 601–610 (2002).
Wang, Q. et al. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol. Cell 27, 380–392 (2007).
Sahu, B. et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 30, 3962–3976 (2011).
Attard, G. et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 27, 3742–3748 (2009).
Acknowledgements
V.K.A. is funded by a Young Investigator Award from the Prostate Cancer Foundation and a Physician Research Training Award from the Department of Defense (W81XWH-11-1-0274). C.L.S. is funded by the Howard Hughes Medical Institute (SU2C/AACR (DT0712), by grants from the US National Cancer Institute (NCI) of the National Institutes of Health (NIH) (R01 CA155169-04, R01 CA19387-01 and T32 CA160001-05), NIH/NCI/Memorial Sloan Kettering Cancer Center (MSKCC) Spore in Prostate Cancer (P50 CA092629-14), and from the NCI/MSKCC Support Grant/Core Grant (P30 CA008748-49 and P30 CA008748-49 S2).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
P.A.W. owns stock in Tokai Pharmaceuticals. C.L.S. is an inventor of patents covering enzalutamide and ARN-509 and is entitled to royalties. He also serves on the Board of Directors of Novartis. V.K.A. declares no competing interests.
Related links
Glossary
- Prostate-specific antigen
-
(PSA). An androgen-regulated serine protease encoded by the gene KLK3, PSA is produced by epithelial cells of the normal and cancerous prostate. Serum levels of PSA are widely used in the clinic as a screening tool for prostate cancer, as well as to monitor cancer recurrence in the post-treatment setting.
- Androgens
-
Male sex steroid hormones, of which testosterone and dihydrotestosterone (DHT) are the principal examples, that bind to and activate the androgen receptor.
- Gonadotropin-releasing hormone
-
(GnRH). Additionally known as luteinizing hormone-releasing hormone (LHRH), GnRH is a small peptide hormone produced in the hypothalamus that stimulates the secretion of luteinizing hormone and follicle-stimulating hormone by the pituitary gland.
- Luteinizing hormone
-
(LH). Secreted by the pituitary gland in response to stimulation by gonadotropin-releasing hormone, LH in turn stimulates receptors on Leydig cells of the testes, which leads to synthesis and secretion of testosterone.
- CYP17A1
-
(Cytochrome P450 family 17 subfamily A polypeptide 1). CYP17A1 possesses both 17α-hydroxylase and 17, 20-lyase activities and is a key enzyme in the synthesis of steroid hormones.
- Glucocorticoids
-
A class of steroid hormones produced by the adrenal gland that are involved in the regulation of metabolism and possess anti-inflammatory activity. The physiological effects of glucocorticoids are mediated through the glucocorticoid receptor.
- Docetaxel
-
An antineoplastic taxane that disrupts microtubule disassembly, resulting in inhibition of mitosis. Docetaxel is approved for use in men with metastatic castration-resistant prostate cancer by the US Food and Drug Administration (FDA).
- ChIP–seq
-
(Chromatin immunoprecipitation followed by sequencing). A technique to ascertain the cistrome of a transcription factor of interest through the use of immunoprecipitation followed by massive parallel sequencing.
- Cistrome
-
The collection of DNA elements within a genome that are bound by a transcription factor.
- Neuroendocrine
-
A rare subtype of prostate cell found in both the normal and cancerous prostate, which is noted for the secretion of numerous neuropeptides.
Rights and permissions
About this article
Cite this article
Watson, P., Arora, V. & Sawyers, C. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 15, 701–711 (2015). https://doi.org/10.1038/nrc4016
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc4016
This article is cited by
-
Integrating single-cell and bulk RNA sequencing data unveils antigen presentation and process-related CAFS and establishes a predictive signature in prostate cancer
Journal of Translational Medicine (2024)
-
Dickkopf-1 (DKK1) drives growth and metastases in castration-resistant prostate cancer
Cancer Gene Therapy (2024)
-
Initial management approach for localized/locally advanced disease is critical to guide metastatic castration-resistant prostate cancer care
Prostate Cancer and Prostatic Diseases (2024)
-
Lysine methyltransferase SMYD2 enhances androgen receptor signaling to modulate CRPC cell resistance to enzalutamide
Oncogene (2024)
-
SC912 inhibits AR-V7 activity in castration-resistant prostate cancer by _targeting the androgen receptor N-terminal domain
Oncogene (2024)