Key Points
-
Lipinski's 'rule of five' has been the leading measure of 'drug-likeness' for many companies since its publication 10 years ago. However, recent trends reveal that the physical properties of molecules that are currently being synthesized are significantly different from oral drugs that have been recently discovered or that are in clinical development.
-
In this article, physicochemical profiles of compounds from patent applications originating from AstraZeneca, GlaxoSmithKline, Merck and Co., and Pfizer are compared with the profiles of recently discovered oral drugs and compounds in development to analyse the specifics of these trends.
-
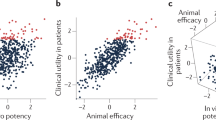
In general, for oral drugs approved since 1983, there has been an increase in molecular mass, O plus N atom count and OH plus NH count, whereas lipophilicity has remained relatively unchanged. We suggest that compound lipophilicity, as estimated by cLogP, is the most important molecular property, as it is changing less over time in launched oral drugs than other properties.
-
However, current medicinal chemistry efforts are producing compounds with higher molecular mass and cLogP than historical oral drugs, recent oral drugs and development compounds. The median patented compound has cLogP of 4.1 and molecular mass of 450 Da, whereas the most recent oral drugs, discovered since 1990, have median cLogP of 3.1 and molecular mass of 432 Da.
-
Although good pharmacokinetic profiles are important in drug development, toxicity is also crucial. It is shown that compound promiscuity is strongly related to lipophilicity. It is suggested that lipophilicity is the most important molecular property to consider for this aspect, as well as high potency, to reduce the risk of toxicity.
-
There is also a broad trend demonstrating that more extreme physical properties and therefore more complex molecules will have concomitantly increased risks to their developability, and thus decreased chances of success as they go through the development process.
-
Although there are many factors influencing the development of a compound, it is proposed that through careful selection of lead compounds and constant monitoring of physical properties (especially lipophilicity) during optimization, medicinal chemists could help alleviate attrition rates.
Abstract
The application of guidelines linked to the concept of drug-likeness, such as the 'rule of five', has gained wide acceptance as an approach to reduce attrition in drug discovery and development. However, despite this acceptance, analysis of recent trends reveals that the physical properties of molecules that are currently being synthesized in leading drug discovery companies differ significantly from those of recently discovered oral drugs and compounds in clinical development. The consequences of the marked increase in lipophilicity — the most important drug-like physical property — include a greater likelihood of lack of selectivity and attrition in drug development. Tackling the threat of compound-related toxicological attrition needs to move to the mainstream of medicinal chemistry decision-making.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Del. Rev. 23, 3–25 (1997).
Lipinski, C. A. Lead- and drug-like compounds: the rule-of-five revolution. Drug Discov. Today Technol. 1, 337–341 (2004). A perspective of the impact of the 'rule of five' in drug discovery.
Abraham, M. H., Chadha, H. S., Whiting, G. S. & Mitchell, R. C. Hydrogen bonding. 32. An analysis of water-octanol and water-alkane partitioning and the Δ log P parameter of Seiler. J. Pharm. Sci. 83, 1085–1100 (1994).
Wenlock, M. C., Austin, R. P., Barton, P., Davis, A. M. & Leeson, P. D. A comparison of physiochemical property profiles of development and marketed oral drugs. J. Med. Chem. 46, 1250–1256 (2003).
Vieth, M. et al. Characteristic physical properties and structural fragments of marketed oral drugs. J. Med. Chem. 47, 224–232 (2004). The physical property profiles of oral drugs are compared with topical and intravenous drugs.
Proudfoot, J. R. The evolution of synthetic oral drug properties. Bioorg. Med. Chem. Lett. 15, 1087–1090 (2005).
Hou, T., Wang, J., Zhang, W. & Xu, X. ADME evaluation in drug discovery. 7. Prediction of oral absorption by correlation and classification. J. Chem. Inf. Model. 47, 208–218 (2007).
Linnankoski, J., Maekelae, J. M., Ranta, V.-P., Urtti, A. & Yliperttula, M. Computational prediction of oral drug absorption based on absorption rate constants in humans. J. Med. Chem. 49, 3674–3681 (2006).
Veber, D. F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 (2002).
Lu, J. J. et al. Influence of molecular flexibility and polar surface area metrics on oral bioavailability in the rat. J. Med. Chem. 47, 6104–6107 (2004).
Martin, Y. C. A bioavailability score. J. Med. Chem. 48, 3164–3170 (2005).
Hou, T., Wang, J., Zhang, W. & Xu, X. ADME evaluation in drug discovery. 6. can oral bioavailability in humans be effectively predicted by simple molecular property-based rules? J. Chem. Inf. Model. 47, 460–463 (2007).
Morphy, R. The influence of _target family and functional activity on the physicochemical properties of pre-clinical compounds. J. Med. Chem. 49, 2969–2978 (2006). A thorough study from the recent literature of medicinal chemical optimization practice across _target classes, and consequent changes in physical-property profiles.
Oprea, T. I. Current trends in lead discovery: are we looking for the appropriate properties? J. Comp Aided Mol. Design 16, 325–334 (2002).
Vieth, M. & Sutherland, J. J. Dependence of molecular properties on proteomic family for marketed oral drugs. J. Med. Chem. 49, 3451–3453 (2006).
Paolini, G. V., Shapland, R. H. B., van Hoorn, W. P., Mason, J. S. & Hopkins, A. L. Global mapping of pharmacological space. Nature Biotech. 24, 805–815 (2006). Exemplifies the power of knowledge exploitation using large databases, providing insights into drug-like chemical space encompassing several hundred proteins.
Leeson, P. D. & Davis, A. M. Time-related differences in the physical property profiles of oral drugs. J. Med. Chem. 47, 6338–6348 (2004).
Blake, J. F. Identification and evaluation of molecular properties related to preclinical optimization and clinical fate. Med. Chem. 1, 649–655 (2005).
Cheng, A. C. et al. Structure-based maximal affinity model predicts small-molecule druggability. Nature Biotech. 25, 71–75 (2007). Accurate 'druggability' predictions emerge from considerations of the predicted affinities of ligands for structurally characterized binding sites.
Hajduk, P. J., Huth, J. R. & Fesik, S. W. Druggability indices for protein _targets derived from NMR-based screening data. J. Med. Chem. 48, 2518–2525 (2005).
Booth, B. & Zemmel, R. Prospects for productivity. Nature Rev. Drug Discov. 3, 451–456 (2004).
van de Waterbeemd, H., Smith, D. A., Beaumont, K. & Walker, D. K. Property-based design: optimization of drug absorption and pharmacokinetics. J. Med. Chem. 44, 1313–1333 (2001). A key paper, laying down the essential principles and importance of physical-property optimization.
Hansch, C. Quantitative approach to biochemical structure–activity relationships. Acc. Chem. Res. 2, 232–9 (1969).
Tute, M. S. Principles and practice of Hansch analysis. Guide to structure–activity correlation for the medicinal chemist. Adv. Drug Res. 6, 1–77 (1971).
Kubinyi, H. Lipophilicity and biological activity. Drug transport and drug distribution in model systems and in biological systems. Arzn. Forsch. 29, 1067–1080 (1979).
Cronin, M. T. D. The role of hydrophobicity in toxicity prediction. Curr. Comp. Aided Drug Des. 2, 405–413 (2006).
Krejsa, C M. et al. Predicting ADME properties and side effects: the BioPrint approach. (http://www.cerep.fr/cerep/users/pages/collaborations/bioprint.asp). Curr. Opin. Drug Disc. Dev. 6, 470–480 (2003).
Fliri, A. F., Loging, W. T., Thadeio, P. F. & Volkmann, R. A. Analysis of drug-induced effect patterns to link structure and side effects of medicines. Nature Chem. Biol. 1, 389–397 (2005). One of the first studies showing that in vitro binding profiles across multiple assays can be used to predict clinical side effects.
Hopkins, A. L., Mason, J. S. & Overington, J. P. Can we rationally design promiscuous drugs? Curr. Opin. Struct. Biol. 16, 127–136 (2006).
Morphy, R. & Rankovic, Z. The physicochemical challenges of designing multiple ligands. J. Med. Chem. 49, 4961–4970 (2006).
Price, D. A. et al. Overcoming HERG affinity in the discovery of the CCR5 antagonist maraviroc. Bioorg. Med. Chem. Lett. 16, 4633–4637 (2006).
Cumming, J. G. et al. Modulators of the human CCR5 receptor. SAR of substituted 1-[3-(4-methanesulfonylphenyl)-3-phenylpropyl]-piperidinyl phenylacetamides. Bioorg. Med. Chem. Lett. 16, 3533–3536 (2006).
Kim, D. et al. Potent 1,3,4-trisubstituted pyrrolidine CCR5 receptor antagonists: effects of fused heterocycles on antiviral activity and pharmacokinetic properties. Bioorg. Med. Chem. Lett. 15, 2129–2134 (2005).
Kazmierski, W. M. et al. CCR5 Antagonists as Therapeutic Agents. World Patent WO2004054974 (2004).
Waring, M. J. & Johnstone, C. A quantitative assessment of hERG liability as a function of lipophilicity. Bioorg. Med. Chem. Lett. 17, 1759–1764 (2007).
Baciu, M. et al. Degradative transport of cationic amphiphilic drugs across phospholipid bilayers. Philos. Trans. A. Math. Phys. Eng. Sci. 364, 2597–2614 (2006).
Leach, A. R., Hann, M. M., Burrows, J. N. & Griffen, E. J. Fragment screening: an introduction. Mol. Biosyst. 2, 429–446 (2006).
Uetrecht, J. Prediction of a new drug's potential to cause idiosyncratic reactions. Curr. Opin. Drug Disc. Devel. 4, 55–59 (2001).
van de Waterbeemd, H. & Gifford, E. ADMET in silico modelling: towards prediction paradise? Nature Rev. Drug Discov. 2, 192–204 (2003).
van de Waterbeemd, H. & Jones, B. C. Predicting oral absorption and bioavailability. Prog. Med. Chem. 41, 1–59 (2003).
Lobell, M. et al. In silico ADMET traffic lights as a tool for the prioritization of HTS hits. ChemMedChem 1, 1229–1236 (2006).
Wunberg, T. et al. Improving the hit-to-lead process: data-driven assessment of drug-like and lead-like screening hits. Drug Discov. Today 11, 175–180 (2006).
Davis, A. M., Keeling, D. J., Steele, J., Tomkinson, N. P. & Tinker, A. C. Components of successful lead generation. Curr. Top. Med. Chem. 5, 421–439 (2005).
Steinmeyer, A. The hit-to-lead process and Schering AG: strategic aspects. ChemMedChem 6, 1–7, (2005).
Bleicher, K. H., Nettekoven, M., Peters, J.-U. & Wyler, R. Lead generation: sowing the seeds for future success. Chimia 58, 588–600 (2004).
Jacoby, E. et al. Key aspects of the Novartis Compound Collection Enhancement Project for the compilation of a comprehensive chemogenomics drug discovery screening collection. Curr. Top. Med. Chem. 5, 397–411 (2005).
Teague, S J., Davis, A. M., Leeson, P. D. & Oprea, T. The design of leadlike combinatorial libraries. Angew. Chem. Int. Ed. 38, 3743–3748 (1999). The introduction of the 'lead-like' concept.
Makara, G. M. On sampling of fragment space. J. Med. Chem. 50, 3214–3221 (2007).
Hann, M. M., Leach, A. R. & Harper, G. Molecular complexity and its impact on the probability of finding leads for drug discovery. J. Chem. Inf. Comp. Sci. 41, 856–864 (2001). A fundamental paper, showing that increased molecular complexity reduces the probability of binding.
Oprea, T. I., Davis, A. M., Teague, S. J. & Leeson, P. D. Is there a difference between leads and drugs? A historical perspective. J. Chem. Inf. Comp. Sci. 41, 1308–1315 (2001).
Hopkins, A. L. & Polinsky, A. Knowledge and intelligence in drug design. Annu. Rep. Med. Chem. 41, 425–437 (2006). An interesting review discussing how medicinal chemists use their knowledge to make choices in drug discovery projects.
Lombardino, J. G. & Lowe, J. A. A guide to drug discovery: the role of the medicinal chemist in drug discovery — then and now. Nature Rev. Drug Discov. 3, 853–862 (2004).
MacCoss, M. & Baillie, T. A. Organic chemistry in drug discovery. Science 303, 1810–1813 (2004).
Leeson, P. D., Davis, A. M. & Steele, J. Drug-like properties: guiding principles for design – or chemical prejudice? Drug Discov. Today Technol. 1, 189–195 (2004).
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nature Rev. Drug Discov. 3, 711–716 (2004).
Food and Drug Administration (FDA). Approved Drug Products with Therapeutic Equivalence Evaluations (FDA Orange Book). FDA web site [online], (2007).
Science Integrity®. http://integrity.prous.com © Prous Science, a Thomson Scientific business; all rights reserved (2007).
Wood, A. Annual Reports in Medicinal Chemistry Volume 41 503–522 (Academic Press, Burlington, 2006).
American Chemical Society. Chemical Abstract Database (CAS): Scifinder. CAS web site [online], (2007).
Merck Research Laboratories. The Merck Index. Cambridge Soft web site [online], (2007).
Gray, N. Changing Landscapes: A Special Report on the World's Top 50 Pharma Companies. Pharmaceutical Executive web site [online], (2006)
GVK Bio. GVK Bio databases. GVK Bio web site [online], (2007).
Beaumont, K., Schmid, E. & Smith, D. A. Oral delivery of G protein-coupled receptor modulators: an explanation for the observed class difference. Bioorg. Med. Chem. Lett. 15, 3658–3664 (2005).
SAS Institute. JMP Statistical Software. SAS Institute web site [online], (2007)
TIBCO Software Inc. Spotfire DecisionSite product suite. TIBCO Software web site [online], (2007).
Azzaoui, K. et al. Modeling promiscuity based on in vitro safety pharmacology profiling data. ChemMedChem 2, 874–880 (2007).
Bender, A. et al. Analysis of pharmacology data and the prediction of adverse drug reactions and off-_target effects from chemical structure. ChemMedChem 2, 861–873 (2007).
Acknowledgements
We gratefully acknowledge numerous fruitful discussions on physical properties with AstraZeneca colleagues S. Boyer, D. Cheshire, A. Davis, J. Dixon, D. Lathbury, J. Li, J.-E. Nyström, G. Pairaudeau, M. Rolf (who provided BioPrint data), J. Steele, S. Teague and M. Wenlock (who helped to assemble the oral drugs database). J. Proudfoot (Boehringer Ingelheim Pharmaceuticals) very kindly provided his oral drug database.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary information S1 (table)
The results of straight-line fits of mean property versus year for oral drugs launched from 1983-2006 (PDF 127 kb)
Supplementary information S2 (box)
Molecular weight (Mol wt) versus publication date for drug classes with common pharmacophores. (PDF 319 kb)
Supplementary information S3 (box)
Plots of promiscuity versus cLogP for Cerep Bioprint data, according to ionization type. (PDF 425 kb)
Supplementary information S4 (table)
Numbers of compounds or patents in _target classes by company and source (GVK Bio Patents, GVK Bio Compounds, Prous Integrity and 1983-2007 oral drugs (PDF 208 kb)
Supplementary information S5 (box)
Properties of oral drugs launched 1983-2007: cLogP. (PDF 5842 kb)
Related links
Glossary
- LogP
-
Log of the octanol–water partition coefficient, which is a measure of a drug's lipophilicity. Defined as the ratio of un-ionized drug distributed between the octanol and water phases at equilibrium. Higher values imply greater lipophilicity.
- Molecular mass
-
The molecular mass of a substance, frequently called molecular weight, is the mass of one molecule of that substance, and its units are the unified atomic mass unit (u) or Dalton (Da), which equals 1/12 the mass of one atom of carbon-12.
- Polar surface area
-
(PSA). This is defined as the surface sum over all polar atoms, (usually oxygen and nitrogen), also including attached hydrogens.
- Bioavailability
-
This is the fraction of an oral dose that reaches the systemic circulation.
- Chemical space
-
This is the space spanned by all energetically stable stoichiometric combinations of electrons, atomic nuclei and topologies in molecules. Drug-like space may contain 1 × 1020 to 1 × 10200 molecules. All these molecules can never be made — to date 2.7 × 107 molecules have been reported.
- Pharmacophore
-
The ensemble of steric and electronic features that is necessary to ensure optimal interactions with a specific biological _target structure and to trigger (or to block) its biological response.
- Phospholipidosis
-
Phospholipidosis is a lipid storage disorder in which excess phospholipids accumulate within cells. Drug-induced phospholipidosis occurs with many cationic amphiphilic drugs.
Rights and permissions
About this article
Cite this article
Leeson, P., Springthorpe, B. The influence of drug-like concepts on decision-making in medicinal chemistry. Nat Rev Drug Discov 6, 881–890 (2007). https://doi.org/10.1038/nrd2445
Issue Date:
DOI: https://doi.org/10.1038/nrd2445
This article is cited by
-
Reversed-phase thin-layer chromatography and ultra-performance liquid chromatography/mass spectrometry to estimate the drug likeness of phosphodiesterase 10A inhibitors with phthalimide core
JPC – Journal of Planar Chromatography – Modern TLC (2024)
-
Advances in Metal-Catalyzed Denitrogenative Pathways for N-Heterocycle Synthesis
Topics in Catalysis (2024)
-
Exploring the latest breakthroughs in rhodesain inhibitors for African trypanosomiasis
Medicinal Chemistry Research (2024)
-
Miniaturization of popular reactions from the medicinal chemists’ toolbox for ultrahigh-throughput experimentation
Nature Synthesis (2023)
-
Extracting medicinal chemistry intuition via preference machine learning
Nature Communications (2023)