Key Points
-
A fitness landscape relates the genotype of an organism to its reproductive capacity and therefore has a central role in evolutionary biology.
-
Introduced in the 1930s, the fitness landscape concept has long been used primarily as a metaphor. This has recently changed, as new experimental tools allow the systematic construction and analysis of combinations of predefined sets of genetic mutations.
-
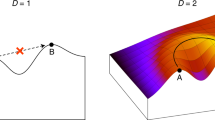
The topography of the fitness landscape is determined by how different mutations interact in their effect on fitness. A particular type of epistasis known as sign epistasis causes the fitness landscape to be rugged, possibly with multiple peaks.
-
A survey of experimental studies shows that most empirical fitness landscapes are rugged, but the amount of ruggedness varies systematically depending on the way the mutations that form the landscape have been chosen.
-
On rugged fitness landscapes, the accessibility of mutational pathways towards higher fitness is reduced, which makes the evolutionary process more constrained and hence predictable. In addition, predictability depends on population size in ways that can be explored using mathematical modelling.
-
A key challenge for the future is to extend current fitness landscape studies to genome-wide scales and to develop models that are informed by the interactions of biomolecules.
Abstract
The genotype–fitness map (that is, the fitness landscape) is a key determinant of evolution, yet it has mostly been used as a superficial metaphor because we know little about its structure. This is now changing, as real fitness landscapes are being analysed by constructing genotypes with all possible combinations of small sets of mutations observed in phylogenies or in evolution experiments. In turn, these first glimpses of empirical fitness landscapes inspire theoretical analyses of the predictability of evolution. Here, we review these recent empirical and theoretical developments, identify methodological issues and organizing principles, and discuss possibilities to develop more realistic fitness landscape models.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
We are sorry, but there is no personal subscription option available for your country.
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lehner, B. Genotype to phenotype: lessons from model organisms for human genetics. Nature Rev. Genet. 14, 168–178 (2013).
Wagner, G. P. & Zhang, J. The pleiotropic structure of the genotype–phenotype map: the evolvability of complex organisms. Nature Rev. Genet. 12, 204–213 (2011).
Phillips, P. C. Epistasis — the essential role of gene interactions in the structure and evolution of genetic systems. Nature Rev. Genet. 9, 855–867 (2008).
de Visser, J. A. G. M., Cooper, T. F. & Elena, S. F. The causes of epistasis. Proc. R. Soc. B 278, 3617–3624 (2011).
Gavrilets, S. Fitness Landscapes and the Origin of Species (Princeton Univ. Press, 2004).
Achaz, G., Rodriguez-Verdugo, A., Gaut, B. S. & Tenaillon, O. The reproducibility of adaptation in the light of experimental evolution with whole genome sequencing. Adv. Exp. Med. Biol. 781, 211–231 (2014).
Lobkovsky, A. E. & Koonin, E. V. Replaying the tape of life: quantification of the predictability of evolution. Frontiers Genet. 3, 246 (2012).
Wright, S. The roles of mutation, inbreeding, crossbreeding and selection in evolution. Proc. 6th Int. Congress Genet. 1, 356–366 (1932). This paper introduces the concept of the fitness landscape as a key component of Wright's shifting balance theory.
Colegrave, N. & Buckling, A. Microbial experiments on adaptive landscapes. BioEssays 27, 1167–1173 (2005).
Szendro, I. G., Schenk, M. F., Franke, J., Krug, J. & de Visser, J. A. G. M. Quantitative analyses of empirical fitness landscapes. J. Stat. Mech. P01005 (2013).
Wright, S. Evolution in Mendelian populations. Genetics 16, 97–159 (1931).
Haldane, J. B. S. A mathematical theory of natural selection. Part VIII. Metastable populations. Proc. Cambridge Philos. Soc. 27, 137–142 (1931).
Maynard Smith, J. Natural selection and the concept of a protein space. Nature 225, 563–564 (1970). This study presents the realization that genotypic space is discrete and that mutational pathways are only accessible when they pass through functional genotypes.
Kauffman, S. A. & Levin, S. Towards a general theory of adaptive walks on rugged landscapes. J. Theor. Biol. 128, 11–45 (1987). This is the first mathematical exploration of random fitness landscapes and their consequences for adaptation.
Kauffman, S. A. & Weinberger, E. D. The NK model of rugged fitness landscapes and its application to the maturation of the immune response. J. Theor. Biol. 141, 211–245 (1989).
Harms, M. J. & Thornton, J. W. Evolutionary biochemistry: revealing the historical and physical causes of protein properties. Nature Rev. Genet. 14, 559–571 (2013).
Malcolm, B. A., Wilson, K. P., Matthews, B. W., Kirsch, J. F. & Wilson, A. C. Ancestral lysozymes reconstructed, neutrality tested, and thermostability linked to hydrocarbon packing. Nature 345, 86–89 (1990). This is the first empirical analysis of a three-locus fitness landscape of lysozymes in game birds.
Barrick, J. E. & Lenski, R. E. Genome dynamics during experimental evolution. Nature Rev. Genet. 14, 827–839 (2013).
Kondrashov, A. S. Deleterious mutations and the evolution of sexual reproduction. Nature 336, 435–440 (1988).
Kouyos, R. D., Silander, O. K. & Bonhoeffer, S. Epistasis between deleterious mutations and the evolution of recombination. Trends Ecol. Evol. 22, 308–315 (2007).
de Visser, J. A. G. M., Hoekstra, R. F. & van den Ende, H. Test of interaction between genetic markers that affect fitness in Aspergillus niger. Evolution 51, 1499–1505 (1997).
Hall, D. W., Agan, M. & Pope, S. C. Fitness epistasis among 6 biosynthetic loci in the budding yeast Saccharomyces cerevisiae. J. Hered. 101, S75–S84 (2010).
Kondrashov, F. A. & Kondrashov, A. S. Multidimensional epistasis and the disadvantage of sex. Proc. Natl Acad. Sci. USA 98, 12089–12092 (2001).
Weinreich, D. M., Watson, R. A. & Chao, L. Perspective: sign epistasis and genetic constraint on evolutionary trajectories. Evolution 59, 1165–1174 (2005). This paper formally introduces the concept of sign epistasis and proves its equivalence with limited pathway accessibility.
Poelwijk, F. J., Tanase-Nicola, S., Kiviet, D. J. & Tans, S. J. Reciprocal sign epistasis is a necessary condition for multi-peaked fitness landscapes. J. Theor. Biol. 272, 141–144 (2011).
Weinreich, D. M., Delaney, N. F., DePristo, M. A. & Hartl, D. L. Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312, 111–114 (2006). This seminal study shows how sign epistasis limits the number of accessible trajectories on a five-locus fitness landscape of β-lactamase.
Weinreich, D. M., Lan, Y., Wylie, S. C. & Heckendorn, R. B. Should evolutionary geneticists worry about higher-order epistasis? Curr. Opin. Genet. Dev. 23, 700–707 (2013).
O'Maille, P. E. et al. Quantitative exploration of the catalytic landscape separating divergent plant sesquiterpene synthases. Nature Chem. Biol. 4, 617–623 (2008).
Lee, Y.-H., Dsouza, L. M. & Fox, G. E. Equally parsimonious pathways through an RNA sequence space are not equally likely. J. Mol. Evol. 45, 278–284 (1997).
Aita, T., Iwakura, M. & Husimi, Y. A cross-section of the fitness landscape of dihydrofolate reductase. Protein Engineer. 14, 633–638 (2001).
Bridgham, J. T., Carroll, S. M. & Thornton, J. W. Evolution of hormone-receptor complexity by molecular exploitation. Science 312, 97–101 (2006).
Brown, K. M. et al. Compensatory mutations restore fitness during the evolution of dihydrofolate reductase. Mol. Biol. Evol. 27, 2682–2690 (2010).
da Silva, J., Coetzer, M., Nedellec, R., Pastore, C. & Mosier, D. E. Fitness epistasis and constraints on adaptation in a human immunodeficiency virus type 1 protein region. Genetics 185, 293–303 (2010).
Goulart, C. P. et al. Designing antibiotic cycling strategies by determining and understanding local adaptive landscapes. PLoS ONE 8, e56040 (2013).
Lozovsky, E. R. et al. Stepwise acquisition of pyrimethamine resistance in the malaria parasite. Proc. Natl Acad. Sci. USA 106, 12015–12030 (2009).
Lunzer, M., Miller, S. P., Felsheim, R. & Dean, A. M. The biochemical architecture of an ancient adaptive landscape. Science 310, 499–501 (2005). This study reconstructs a fitness landscape by analysing enzyme function as a phenotype that links genotype and fitness.
Novais, A. et al. Evolutionary trajectories of β-lactamase CTX-M-1 cluster enzymes: predicting antibiotic resistance. PLoS Pathog. 6, e1000735 (2010).
Tan, L., Serene, S., Chao, H. X. & Gore, J. Hidden randomness between fitness landscapes limits reverse evolution. Phys. Rev. Lett. 106, 198102 (2011).
de Vos, M. G. J., Poelwijk, F. J., Battich, N., Ndika, J. D. T. & Tans, S. J. Environmental dependence of genetic constraint. PLoS Genet. 9, e1003580 (2013).
Chou, H.-H., Chiu, H.-C., Delaney, N. F., Segrè, D. & Marx, C. J. Diminishing returns epistasis among beneficial mutations decelerates adaptation. Science 332, 1190–1192 (2011).
Khan, A. I., Dinh, D. M., Schneider, D., Lenski, R. E. & Cooper, T. F. Negative epistasis between beneficial mutations in an evolving bacterial population. Science 332, 1193–1196 (2011).
Franke, J., Klözer, A., de Visser, J. A. G. M. & Krug, J. Evolutionary accessibility of mutational pathways. PLoS Computat. Biol. 7, e1002134 (2011).
Whitlock, M. C. & Bourguet, D. Factors affecting the genetic load in Drosophila: synergistic epistasis and correlations among fitness components. Evolution 54, 1654–1660 (2000).
Schenk, M. F., Szendro, I. G., Salverda, M. L. M., Krug, J. & de Visser, J. A. G. M. Patterns of epistasis between beneficial mutations in an antibiotic resistance gene. Mol. Biol. Evol. 30, 1779–1787 (2013).
Draghi, J. A. & Plotkin, J. B. Selection biases the prevalence and type of epistasis along adaptive trajectories. Evolution 67, 3120–3131 (2013).
Pumir, A. & Shraiman, B. Epistasis in a model of molecular signal transduction. PLoS Comput. Biol. 7, e1001134 (2011).
Wilke, C. O. & Adami, C. Interaction between directional epistasis and average mutational effects. Proc. R. Soc. B 268, 1469–1474 (2001).
You, L. & Yin, J. Dependence of epistasis on environment and mutation severity as revealed by in silico mutagenesis of phage T7. Genetics 160, 1273–1281 (2002).
DePristo, M. A., Weinreich, D. M. & Hartl, D. L. Missense meanderings in sequence space: a biophysical view of protein evolution. Nature Rev. Genet. 6, 678–687 (2005).
Watson, R. A., Weinreich, D. M. & Wakeley, J. Genome structure and the benefits of sex. Evolution 65, 523–536 (2010).
Conway Morris, S. Life's Solution: Inevitable Humans in a Lonely Universe (Cambridge Univ. Press, 2003).
Gould, S. J. Wonderful Life: The Burgess Shale and the Nature of History (W. W. Norton & Company, 1989).
Lang, G. I. et al. Pervasive genetic hitchhiking and clonal interference in forty evolving yeast populations. Nature 500, 571–574 (2013).
Tenaillon, O. et al. The molecular diversity of adaptive convergence. Science 335, 457–461 (2012).
Woods, R., Schneider, D., Winkworth, C. L., Riley, M. A. & Lenski, R. E. Tests of parallel molecular evolution in a long-term experiment with Escherichia coli. Proc. Natl Acad. Sci. USA 103, 9107–9112 (2006).
Blount, Z. D., Barrick, J. E., Davidson, C. J. & Lenski, R. E. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature 489, 513–518 (2012).
Salverda, M. L. M. et al. Initial mutations direct alternative pathways of protein evolution. PLoS Genet. 7, e1001321 (2011).
Papp, B., Notebaart, R. A. & Pál, C. Systems-biology approaches for predicting genomic evolution. Nature Rev. Genet. 12, 591–602 (2011).
Gerrish, P. J. & Sniegowski, P. D. Real time forecasting of near-future evolution. J. R. Soc. Interface 9, 2268–2278 (2012).
Gillespie, J. H. Some properties of finite populations experiencing strong selection and weak mutation. Am. Naturalist 121, 691–708 (1983).
Orr, H. A. The genetic theory of adaptation: a brief history. Nature Rev. Genet. 6, 119–127 (2005).
Crona, K., Greene, D. & Barlow, M. The peaks and geometry of fitness landscapes. J. Theor. Biol. 317, 1–10 (2013).
Whitlock, M. C., Phillips, P. C., Moore, F. B.-G. & Tonsor, S. J. Multiple fitness peaks and epistasis. Annu. Rev. Ecol. Systemat. 26, 601–629 (1995).
Hegarty, P. & Martinsson, A. On the existence of accessible paths in various models of fitness landscapes. Ann. Appl. Prob. (in the press).
Schmiegelt, B. & Krug, J. Evolutionary accessibility of modular fitness landscapes. J. Statist. Phys. 154, 334–355 (2014).
Roy, S. W. Probing evolutionary repeatability: neutral and double changes and the predictability of evolutionary adaptation. PLoS ONE 4, e4500 (2009).
Gerrish, P. J. & Lenski, R. E. The fate of competing beneficial mutations in an asexual population. Genetica 102–103, 127–144 (1998).
Jain, K., Krug, J. & Park, S.-C. Evolutionary advantage of small populations on complex fitness landscapes. Evolution 65, 1945–1955 (2011).
Rozen, D. E., Habets, M. G. J. L., Handel, A. & de Visser, J. A. G. M. Heterogeneous adaptive trajectories of small populations on complex fitness landscapes. PLoS ONE 3, e1715 (2008).
Weissman, D. B., Desai, M. M., Fisher, D. S. & Feldman, M. W. The rate at which asexual populations cross fitness valleys. Theor. Popul. Biol. 75, 286–300 (2009).
Isawa, Y., Michor, F. & Nowak, M. A. Stochastic tunnels in evolutionary dynamics. Genetics 166, 1571–1579 (2004).
Woods, R. J. et al. Second-order selection for evolvability in a large Escherichia coli population. Science 331, 1433–1436 (2011). This study experimentally shows the combined influence of epistasis and population dynamics on the outcome of evolution.
Szendro, I. G., Franke, J., de Visser, J. A. G. M. & Krug, J. Predictability of evolution depends non-monotonically on population size. Proc. Natl Acad. Sci. USA 110, 571–576 (2013).
Rowe, W. et al. Analysis of a complete DNA–protein affinity landscape. J. R. Soc. Interface 7, 397–408 (2010).
Pitt, J. N. & Ferré-D'Amaré, A. R. Rapid construction of empirical RNA fitness landscapes. Science 330, 376–379 (2010).
Jiménez, J. I., Xulvi-Brunet, R., Campbell, G. W., Turk-MacLeod, R. & Chen, I. A. Comprehensive experimental fitness landscape and evolutionary network for small RNA. Proc. Natl Acad. Sci. 110, 14984–14989 (2013). This is an empirical analysis of the largest fitness landscape so far and involves >1014 RNA molecules.
Hinkley, T. et al. A systems analysis of mutational effects in HIV-1 protease and reverse transcriptase. Nature Genet. 43, 487–490 (2011). This paper presents an early empirical fitness landscape of large dimensions for HIV-1 with fitness predictions for the many missing genotypes.
Kouyos, R. D. et al. Exploring the complexity of the HIV-1 fitness landscape. PLoS Genet. 8, e1002551 (2012).
Otwinowski, J. & Nemenman, I. Genotype to phenotype mapping and the fitness landscape of the E. coli lac promoter. PLoS ONE 8, e61570 (2013).
Kinney, J. B., Murugan, A., Callan, C. G. & Cox, E. C. Using deep sequencing to characterize the biophysical mechanism of a transcriptional regulatory sequence. Proc. Natl Acad. Sci. 107, 9158–9163 (2010).
Provine, W. B. Sewall Wright and Evolutionary Biology (Chicago Univ. Press, 1986).
de Visser, J. A. G. M., Park, S.-C. & Krug, J. Exploring the effect of sex on empirical fitness landscapes. Am. Naturalist 174, S15–S30 (2009).
Wagner, A. Neutralism and selectionism: a network-based reconciliation. Nature Rev. Genet. 9, 965–974 (2008).
Hietpas, R. T., Jensen, J. D. & Bolona, D. N. Experimental illumination of a fitness landscape. Proc. Natl Acad. Sci. USA 108, 7896–7901 (2011).
Heckmann, D. et al. Predicting C4 photosynthesis evolution: modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153, 1579–1588 (2013).
Perfeito, L., Ghozzi, S., Berg, J., Schnetz, K. & Lässig, M. Nonlinear fitness landscape of a molecular pathway. PLoS Genet. 7, e1002160 (2011).
Chan, H. S. & Bornberg-Bauer, E. Perspectives on protein evolution from simple exact models. Appl. Bioinformat. 1, 121–144 (2002).
Schuster, P. Prediction of RNA secondary structures: from theory to models and real molecules. Rep. Progress Phys. 69, 1419–1477 (2006).
Mustonen, V., Kinney, J., Callan, C. G. & Lässig, M. Energy-dependent fitness: a quantitative model for the evolution of yeast transcription factor binding sites. Proc. Natl Acad. Sci. 105, 12376–12381 (2008).
Heo, M., Kang, L. & Shakhnovich, E. I. Emergence of species in evolutionary “simulated annealing”. Proc. Natl Acad. Sci. USA 106, 1869–1874 (2009).
Wylie, S. C. & Shakhnovich, E. I. A biophysical protein folding model accounts for most mutational fitness effects in viruses. Proc. Natl Acad. Sci. USA 108, 9916–9921 (2011).
Russell, C. A. et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host. Science 336, 1541–1547 (2012).
Gong, L. I., Suchard, M. A. & Bloom, J. D. Stability-mediated epistasis constrains the evolution of an influenza protein. eLife 2, e00631 (2013).
Hall, B. G. Predicting evolution by in vitro evolution requires determining evolutionary pathways. Antimicrob. Agents Chemother. 46, 3035–3038 (2002).
Palmer, A. C. & Kishony, R. Understanding, predicting and manipulating the genotypic evolution of antibiotic resistance. Nature Rev. Genet. 14, 243–248 (2013).
Ferguson, Andrew, L. et al. Translating HIV sequences into quantitative fitness landscapes predicts viral vulnerabilities for rational immunogen design. Immunity 38, 606–617 (2013).
Hansen, T. F. & Wagner, G. P. Modeling genetic architecture: a multilinear theory of gene interaction. Theor. Popul. Biol. 59, 61–86 (2001).
Neher, R. A. & Shraiman, B. I. Statistical genetics and evolution of quantitative traits. Rev. Modern Phys. 83, 1283–1300 (2011).
Stadler, P. F. & Happel, R. Random field models of fitness landscapes. J. Math. Biol. 38, 435–478 (1999).
Neidhart, J., Szendro, I. G. & Krug, J. Exact results for amplitude spectra of fitness landscapes. J. Theor. Biol. 332, 218–227 (2013).
Kingman, J. F. C. A simple model for the balance between selection and mutation. J. Appl. Probabil. 15, 1–12 (1978).
Lobkovsky, A. E., Wolf, Y. I. & Koonin, E. V. Predictability of evolutionary trajectories in fitness landscapes. PLoS Comput. Biol. 7, e1002302 (2011).
Palmer, M. E., Moudgil, A. & Feldman, M. W. Long-term evolution is surprisingly predictable in lattice proteins. J. R. Soc. Interface 10, 20130026 (2013).
Ferrada, E. & Wagner, A. A comparison of genotype-phenotype maps for RNA and proteins. Biophys. J. 102, 1916–1925 (2012).
Martin, G., Elena, S. F. & Lenormand, T. Distributions of epistasis in microbes fit predictions from a fitness landscape model. Nature Genet. 33, 555–560 (2007).
Rokyta, D. R. et al. Epistasis between beneficial mutations and the phenotype-to-fitness map for a ssDNA virus. PLoS Genet. 7, e1002075 (2011).
Pearson, V. M., Miller, C. R. & Rokyta, D. R. The consistency of beneficial fitness effects of mutations across diverse genetic backgrounds. PLoS ONE 7, e43864 (2012).
Chou, H.-H., Delaney, N. F., Draghi, J. A. & Marx, C. J. Mapping the fitness landscape of gene expression uncovers the cause of antagonism and sign epistasis between adaptive mutations. PLoS Genet. 10, e1004149 (2014).
Orr, H. A. The probability of parallel evolution. Evolution 59, 216–220 (2005).
Acknowledgements
The authors thank I. Szendro for assistance with figure 3. They thank D. Weinreich and G. Achaz for sharing subsequently published manuscripts during the preparation phase of this Review, and four anonymous reviewers for constructive comments. This work was supported by Deutsche Forschungsgemeinschaft within SFB 680 “Molecular Basis of Evolutionary Innovation” and SPP 1590 “Probabilistic Structures in Evolution”.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
Glossary
- Fitness
-
A measure of reproductive success of an organism that determines the change of the corresponding genotypic frequency in the population by natural selection.
- Epistasis
-
Any kind of genetic interaction that leads to a dependence of mutational effects on the genetic background.
- Ruggedness
-
A measure of the complexity of fitness landscapes due to multidimensional epistasis. However, it is often used in a more restricted way to reflect the presence of multiple peaks.
- Magnitude epistasis
-
Epistatic interactions that affect the magnitude but not the sign of mutational effects on fitness.
- Hamming distance
-
The distance between two genotypes measured by the number of mutations in which they differ.
- Unidimensional epistasis
-
A description of epistasis based on the curvature of the relationship between average fitness and the number of mutations.
- Multidimensional epistasis
-
Epistatic interaction that reflects the high-dimensional nature of genotypic space.
- Sign epistasis
-
Epistatic interaction that affects the sign of mutational effects on fitness, such that a given mutation can be deleterious or beneficial depending on genetic background.
- Strong-selection–weak-mutation
-
(SSWM). A regime of population dynamics in which beneficial mutations are sufficiently rare to arise and fix independently, while selection is strong enough to prevent the fixation of deleterious mutations.
- Direct paths
-
Shortest mutational pathways between genotypes, along which the distance to the _target genotype decreases by one in each step. There are d! direct paths between two genotypes at Hamming distance d.
- Adaptive walks
-
Trajectories of monomorphic populations moving through genotypic space in single mutational steps, each of which increases fitness.
- 'Greedy' adaptation
-
An adaptive walk in which the available mutation of largest effect is fixed in each step.
- Stochastic tunnelling
-
A mechanism for the crossing of fitness 'valleys', in which the escape genotype arises by mutation from a small valley population. This mechanism is different from that proposed by Wright for crossing valleys through the fixation of deleterious mutations, which happens only under weak selection.
Rights and permissions
About this article
Cite this article
de Visser, J., Krug, J. Empirical fitness landscapes and the predictability of evolution. Nat Rev Genet 15, 480–490 (2014). https://doi.org/10.1038/nrg3744
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrg3744
This article is cited by
-
Evolution of biological cooperation: an algorithmic approach
Scientific Reports (2024)
-
Environmental modulation of global epistasis in a drug resistance fitness landscape
Nature Communications (2023)
-
Statistically learning the functional landscape of microbial communities
Nature Ecology & Evolution (2023)
-
Geometry of fitness landscapes: peaks, shapes and universal positive epistasis
Journal of Mathematical Biology (2023)
-
Beyond Nature Versus Nurture: the Emergence of Emotion
Affective Science (2023)