There is a common belief that increasing omega-3 intake may prevent and treat both depression and anxiety, and in the USA, long-chain omega-3 (LCn3) intakes are greater from dietary supplements (0.72 g/d eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) than foods (0.41 g/d).Reference Papanikolaou, Brooks, Reider and Fulgoni1 Globally depressive disorders are the third most common cause of years lived with disability in women and fifth in men, and anxiety disorders are eighth and fifteenth, respectively.Reference Kyu, Abate, Abate, Abay, Abbafati and Abbasi2 Lifetime prevalence is 10–17% for anxiety disorders and 10–16% for mood disorders,Reference Demyttenaere, Bruffaerts, Posada-Villa, Gasquet, Kovess and Lepine3,Reference Kessler, Angermeyer, Anthony, De Graaf, Demyttenaere and Gasquet4 with higher rates in people with long-term conditions.Reference Wang, Wu, Lai, Long, Zhang and Li5–Reference Matcham, Rayner, Steer and Hotopf7
The aetiological theories of depression and anxiety suggest concurrent alterations in brain chemistry, environmental stressors and genetic predisposition. Polyunsaturated fatty acids (PUFAs), including LCn3 (mostly from fish), alpha-linolenic acid (ALA; a plant-based omega-3) and omega-6 fatty acids (mostly from vegetable oils) have roles in the synthesis, release, reuptake, degradation and binding of neurotransmitters, and in neural structure and function.Reference Dyall8–Reference Larrieu and Layé10 Neuronal cell membranes contain high levels of DHA (an LCn3). Observational research suggests correlations between low omega-3 or fish consumption and depression,Reference Grosso, Micek, Marventano, Castellano, Mistretta and Pajak11,Reference Thesing, Bot, Milaneschi, Giltay and Penninx12 whereas people with social anxiety disorder have lower erythrocyte membrane omega-3 and higher omega-6/omega-3 ratios than controls, and negative correlations between omega-3 levels and anxiety scores have been observed.Reference Green, Hermesh, Monselise, Marom, Presburger and Weizman13 Thus, increasing omega-3 intake and/or reducing omega-6 intakes may have antidepressant and anxiolytic effects,Reference Su, Matsuoka and Pae9,Reference Appleton, Sallis, Perry, Ness and Churchill14 but reverse causation is highly feasible in that poor mental health may lead to lower quality dietary intake.
Aims
We aimed to assess effects of increasing LCn3, ALA, omega-6 or total PUFA on depression and anxiety in randomised controlled trials (RCTs) of at least 6 months duration.
Method
This systematic review and meta-analysis is part of a series of systematic reviews commissioned by the World Health Organization (WHO) assessing health effects of omega-3, omega-6 and total PUFA.Reference Abdelhamid, Brown, Brainard, Biswas, Thorpe and Moore15–Reference Thorpe, Ajabnoor, Ahmed, Abdelhamid and Hooper23 Its protocol was registered (International Prospective Register of Systematic Reviews identifier: CRD42017056092). Specific methods for this review are discussed below, and detailed methods for the review series are reported elsewhere, including detailed search strategies, list of variables data extracted and the wider database of trials.Reference Hooper, Abdelhamid, Brainard, Deane and Song22 No ethical approval was required for this study.
Criteria for considering studies for this review
Types of studies
We included only RCTs of at least 6 months (24 weeks) duration. The 24-week cut-off reflects metabolic studies suggesting 6 months as the minimum duration of supplementation required to ensure equilibration of LCn3 into most body compartments, including the brain.Reference Browning, Walker, Mander, West, Madden and Gambell24
Types of participants
Participants in included studies had to be adults (aged ≥18 years) who were not pregnant or seriously ill. Participants could have a current or previous diagnosis of clinical depression or anxiety, but this was not necessary.
Types of intervention and comparison
Studies were included where they compared higher with lower omega-3, omega-6 and/or total PUFA intakes. The intervention could consist of advice, foodstuffs or oral supplements (oil, capsules or provided foodstuffs) that aimed to alter omega-3, omega-6 and/or total PUFA intake, or (if no specific aim was stated) achieved a change of ≥10% of baseline intake. Studies were excluded if they examined multiple risk factor interventions on lifestyle or dietary factors other than PUFA. Interventions had to be compared with usual diet, no advice, no supplementation or placebo (as appropriate), or compared raised versus lowered PUFA intake over ≥24 weeks.
Types of outcome measures
Included trials assessed at least one of the primary outcomes (even where these outcomes were not fully reported). Primary outcomes were as follows: risk of depression or anxiety symptoms assessed by formal diagnosis or an appropriate scale, dichotomised to give risk of depression or anxiety in participants without depression or anxiety at baseline; severity of depression or anxiety symptoms as a continuous scale in participants with or without existing depression; and severity of depression or anxiety, or relapse, in those with depression at baseline. Assessment of depression or anxiety did not have to be the main study goal.
Secondary outcomes were as follows: social participation; quality of life; carer stress; healthcare and patient costs; adherence; fidelity; adverse events; withdrawal rates; withdrawals owing to non-adherence, lack of efficacy and/or side effects; and psychosis, suicidality, suicide and self-harm. Secondary outcomes were data-extracted from included studies.
Search methods for identification of studies
We searched Cochrane Central, Medline and EMBASE to 27 April 2017; Clinicaltrials.com and the WHO International Clinical Trials Registry Platform to September 2016; and reassessed all ongoing trials in August 2019. Searches were not limited by language or publication date. We checked included trials of relevant systematic reviews, and wrote to authors of included studies for additional studies and trial data (including unpublished summary outcome data). Full search methods and full text of electronic search strategies are reported in full in our methodology paper.Reference Hooper, Abdelhamid, Brainard, Deane and Song22
Data collection
Study inclusion, data extraction and assessment of risk of bias were conducted independently in duplicate, and disagreements were resolved by discussion or a third reviewer.
Assessment of risk of bias in included studies
We assessed Cochrane risk-of-bias domains,Reference Higgins, Altman, Sterne, Higgins and Green25 and also assessed risk from adherence problems and attention bias specifically for our reviews.Reference Abdelhamid, Brown, Brainard, Biswas, Thorpe and Moore15–Reference Thorpe, Ajabnoor, Ahmed, Abdelhamid and Hooper23 Included trials were judged at low summary risk of bias where randomisation, allocation concealment, blinding of participants, personnel and outcome assessors were adequate (all other trials were at moderate or high risk of bias).
Data synthesis
Main analyses assessed effects of increasing omega-6, LCn3, ALA and mixed PUFA on primary outcomes, using random-effects meta-analysis (as dietary interventions are heterogeneous by their natureReference McKenzie, Herbison and Deeks26) with risk ratio or mean differences in Review Manager, version 5.3.27 Where different scales could be combined the direction of scales was standardised (so lower scores signified lower levels of depression or anxiety) and combined by standardised mean differences.
Sensitivity analyses
Prespecified sensitivity analyses of primary outcomes included fixed-effects meta-analysis, limiting analysis to studies at low summary risk of bias, limiting to studies at low risk for adherence issues and limiting to trials randomising ≥100 participants.
Subgroup analysis and investigation of heterogeneity
Prespecified subgroup analyses were conducted for primary outcomes with eight or more included studies by intervention type, replacement, dose, duration, baseline depression risk (high risk was defined as people with clinically diagnosed depression and/or anxiety, using any diagnostic criteria; medium risk was defined as people with depression or anxiety risk factors, such as a long-term conditions; low risk was defined as all other populations) and antidepressant or antianxiety medication use in ≥50% participants.Reference Hooper, Abdelhamid, Brainard, Deane and Song22 We planned to create subgroups by severity of baseline depression and combined anxiety and depression diagnosis, and by baseline intake of omega-3, omega-6 or total PUFA, but only two trials included participants with diagnosed depressionReference Tajalizadekhoob, Sharifi, Fakhrzadeh, Mirarefin, Ghaderpanahi and Badamchizade28 and baseline intake information was not available in most trials, so was not attempted.
We assessed heterogeneity between trials by I 2. We assessed small study bias by funnel plots, Harbord and Peters test (for dichotomous data) or Egger test (for continuous data),Reference Higgins, Altman, Sterne, Higgins and Green25,Reference Harbord, Egger and Sterne29 where there were ten or more included trials, comparison of random- and fixed-effects analyses and knowledge of missing data.
Interpretation of findings
Effect sizes were interpreted as agreed with the WHO Nutrition Guidance Expert Advisory Group Subgroup on Diet and Health (who commissioned this review as part of a set of work to underpin their dietary guidance) and prespecified for this set of reviews.Reference Hooper, Abdelhamid, Brainard, Deane and Song22 Risk ratios of <0.92 or >1.08 were considered to be relevant clinical effects (risk ratios of 0.92 to 1.08 were considered to be little or no effect), whereas a mean difference between arms of ≥10% of baseline was required for a relevant clinical effect for continuous measures. Outcome data were interpreted by the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) assessment, drafted by L.H., then discussed and agreed with the WHO Nutrition Guidance Expert Advisory Group as elaborated elsewhere.Reference Hooper, Abdelhamid, Brainard, Deane and Song22 Where GRADE suggested data of very low quality, we did not interpret effect sizes. Where data were of low quality, we used the term ‘may’; moderate-quality evidence warranted ‘probably’ in describing effect sizes.
Data availability
All authors have ongoing access to the study data within a shared database. The database for this set of reviews is available in our accepted methods and database paper.Reference Hooper, Abdelhamid, Brainard, Deane and Song22
Results
The search strategy for the wider set of reviews found 364 RCTs (reported in 1020 papers) of omega-3, omega-6 or total PUFA with a duration of at least 6 months.Reference Hooper, Abdelhamid, Brainard, Deane and Song22 From this set, 32 RCTs that assessed outcomes of interest were included in this review (Supplementary Fig. 1 available at https://doi.org/10.1192/bjp.2019.234, for more detail see methods paper).Reference Hooper, Abdelhamid, Brainard, Deane and Song22 Systematic review results, including sensitivity analyses and subgrouping, are provided briefly here and in more detail in Supplementary Text 1.
Characteristics of included studies
Characteristics of included studies and risk of bias are detailed in Supplementary Table 1 and in more detail in our database paperReference Brainard, Jimoh, Deane, Biswas, Donaldson and Maas20. A total of 31 trials (41 470 participants) assessed effects of LCn3,Reference Tajalizadekhoob, Sharifi, Fakhrzadeh, Mirarefin, Ghaderpanahi and Badamchizade28,Reference Kromhout, Giltay and Geleijnse30–Reference Van De Rest, Geleijnse, Kok, van Staveren, Hoefnagels and Beekman61 one assessed effects of ALA (4837 participants)Reference Kromhout, Giltay and Geleijnse30 and one assessed effects of higher total PUFA (4997 participants).Reference Estruch, Ros, Salas-Salvadó, Covas, Corella and Arós62 No trials assessed effects of omega-6 on depression or anxiety.
Participants were recruited with chronic illness or risk factors in 17 trials; memory deficit, cognitive impairment or Alzheimer's disease in six trials; mental health problems in four trials and healthy participants in five trials.
Of the 31 LCn3 trials, most gave supplementary capsules or medicinal oils, two used supplemental foods (enriched margarine and fish sausages),Reference Kromhout, Giltay and Geleijnse30,Reference Hashimoto, Kato, Tanabe, Katakura, Mamun and Ohno40 one provided dietary adviceReference Tuttle, Shuler, Packard, Milton, Daratha and Bibus57 and one provided a combination.Reference Vellas, Carrie, Guyonnet, Touchon, Dantoine and Dartigues43 The ALA trial provided enriched margarine,Reference Kromhout, Giltay and Geleijnse30 and the PUFA trial provided dietary advice plus nuts.Reference Estruch, Ros, Salas-Salvadó, Covas, Corella and Arós62 LCn3 doses ranged from 300 to 3360 mg/d of EPA plus DHAReference Tajalizadekhoob, Sharifi, Fakhrzadeh, Mirarefin, Ghaderpanahi and Badamchizade28,Reference Pratt, Reiffel, Ellenbogen, Naccarelli and Kowey54 ; 12 trial arms assessed doses of ≤1000 mg/d, 13 trial arms assessed doses of 1001–2000 mg/d and seven trial arms assessed doses >2000 mg/d. Control groups received olive, corn or sunflower oils; other fats; other ‘inert’ or ill-defined substances; different dietary advice; foods without omega-3 enrichment or nothing.
Risk of bias of included studies
Risk of bias is itemised by domain and study in Fig. 1. Of the 32 RCTs (33 comparisons including 46 467 randomised participants), 12 were judged to be at low summary risk of bias,Reference Kromhout, Giltay and Geleijnse30,Reference Chew, Clemons, Agron, Launer, Grodstein and Bernstein31,Reference Derosa, Cicero, D'Angelo, Borghi and Maffioli34,Reference Danthiir, Hosking, Burns, Wilson, Nettelbeck and Calvaresi38,Reference Vellas, Carrie, Guyonnet, Touchon, Dantoine and Dartigues43,Reference Yurko-Mauro, McCarthy, Rom, Nelson, Ryan and Blackwell45,Reference Pawelczyk, Grancow-Grabka, Kotlicka-Antczak, Trafalska and Pawelczyk48,Reference Rauch, Schiele, Schneider, Diller, Victor and Gohlke50,Reference Dangour, Allen, Elbourne, Fasey, Fletcher and Hardy51,Reference Sinn, Milte, Street, Buckley, Coates and Petkov55,Reference Galan, Kesse-Guyot, Czernichow, Briancon, Blacher and Hercberg56,59,Reference Andrieu, Guyonnet, Coley, Cantet, Bonnefoy and Bordes60 including 12 LCn3 comparisons, and the single ALA assessment (Fig. 1). Trial authors provided some response to attempted contact for 16 trials.
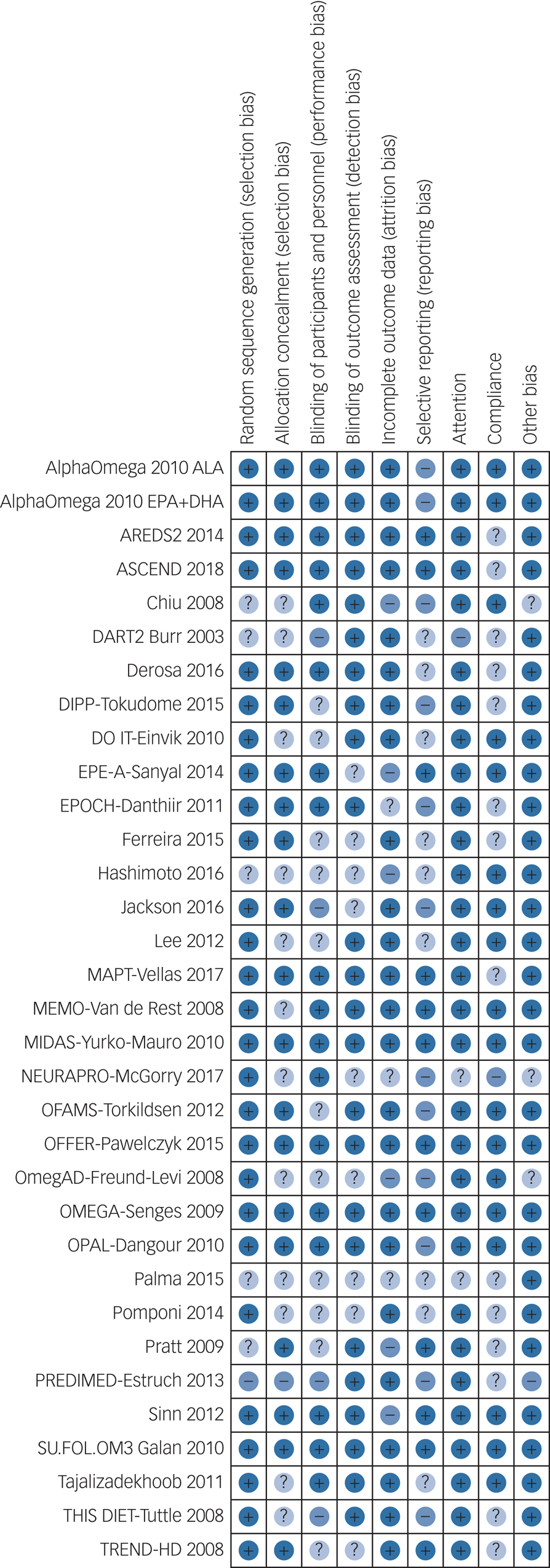
Fig. 1 Itemised risk of bias for included randomised controlled trials.
Effects of increasing omega-3, omega-6 or total PUFA on risk of depression symptoms
Thirteen RCTs (randomising 26 528 participants, reporting 1355 people developing depression symptoms, median dose 0.95 g/d, range 0.4–3.4 g/d, median duration 12 months, range 6–89 months) suggested little or no effect of increasing LCn3 on risk of depression symptoms (RR 1.01, 95% CI 0.92–1.10, I 2 = 0%, Fig. 2). This did not differ in sensitivity analyses by summary risk of bias, fixed effects or study size, although retaining only trials with good adherence suggested increased depression risk with increased LCn3 (RR 1.16, 95% CI 0.99–1.36, I 2 = 0%, Supplementary Table 2). Over 90% of meta-analytic weight came from three trials that assessed depression symptoms dichotomously with the Center for Epidemiologic Studies Depression Scale (score ≥16),Reference Chew, Clemons, Agron, Launer, Grodstein and Bernstein31 Beck Depression Inventory-II (score ≥14)Reference Rauch, Schiele, Schneider, Diller, Victor and Gohlke50 and General Health Questionnaire-30 (score ≥5).Reference Dangour, Allen, Elbourne, Fasey, Fletcher and Hardy51 In other trials depression events were based on Geriatric Depression Scale-15 (score >10) scores, were reported as adverse events or were unclear. There was no suggestion of publication bias in visual inspection of the funnel plot, or with statistical tests (Harbord test P = 0.27, Peters test P = 0.29), and no suggestion of heterogeneity. Effects did not differ by intervention type, replacement nutrients or LCn3 dose, but subgrouping suggested increased depression risk with LCn3 in healthy adults, and little or no effect in those with comorbid illnesses. One LCn3 trial recruited only participants with current depression in which ≥50% took antidepressants.Reference Tajalizadekhoob, Sharifi, Fakhrzadeh, Mirarefin, Ghaderpanahi and Badamchizade28 As prespecified LCn3 dose subgroupings did not divide included trials effectively, post hoc we re-ran even LCn3, EPA and DHA dose subgroupings. There was no suggestion of LCn3 dose effects (test for subgroup differences P = 0.98), EPA (P = 0.13) or DHA (P = 0.87) effects (Supplementary Figs 2–4).
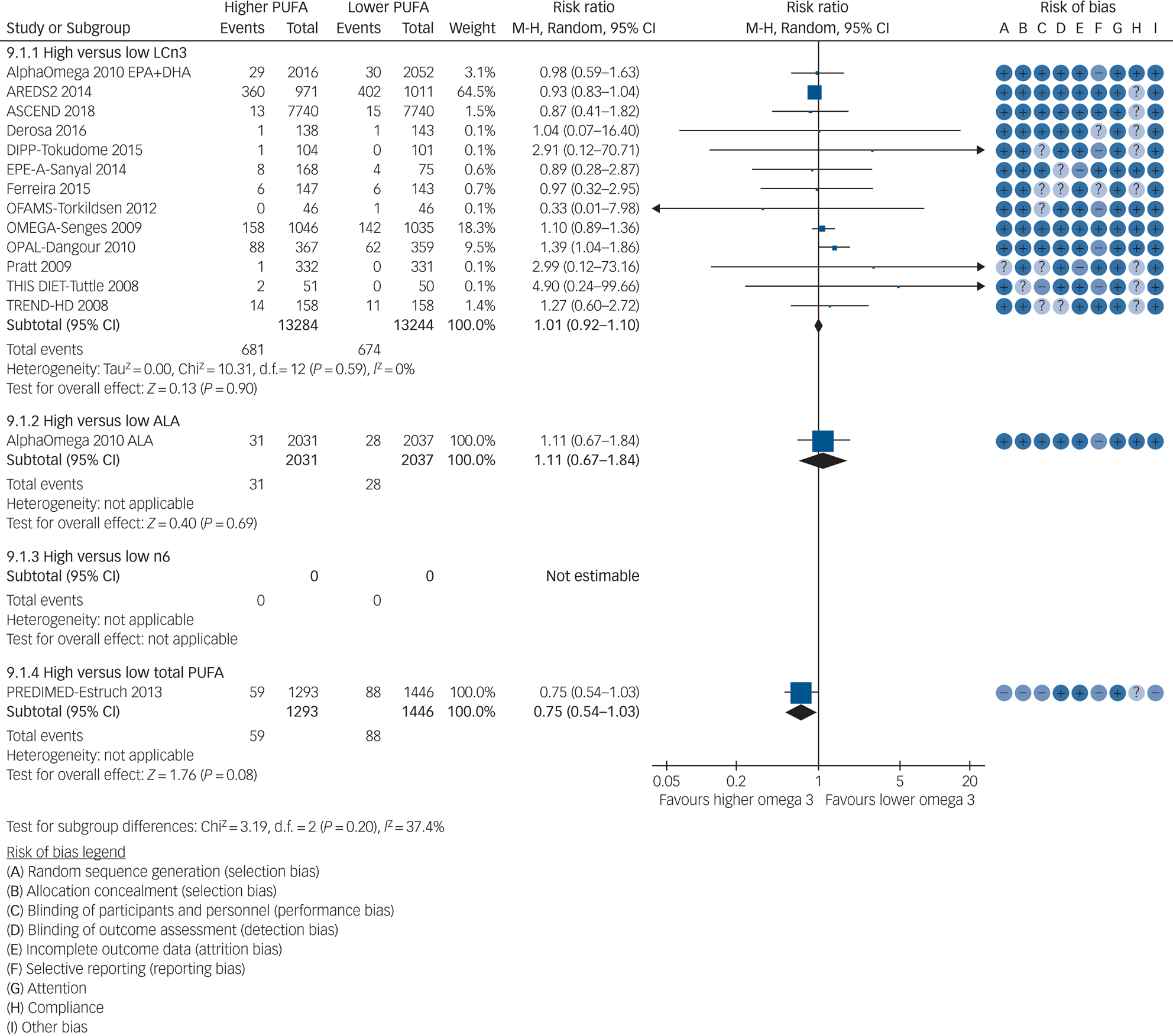
Fig. 2 Meta-analysis of trials randomising to higher versus lower long-chain omega-3 (LCn3), alpha-linolenic acid (ALA), omega-3 and total polyunsaturated fatty acid (PUFA) intake and reporting risk of depression symptoms.
GRADE assessment suggests that increasing LCn3 probably has little or no effect on risk of depression symptoms (moderate-quality evidence, downgraded once for imprecision, Supplementary Table 3). This was confirmed in data on depression symptoms analysed as continuous data in 15 trials including participants not selected for depression at baseline (for details see Supplementary Text 1 and Supplementary Table 3).
Data were limited from trials of ALA and total PUFA on depression (Supplementary Tables 4 and 5). We found no data from trials of omega-6 (Fig. 2). GRADE suggests that increasing ALA may increase risk of depression symptoms very slightly (number needed to harm, 1000; low-quality evidence, downgraded twice for imprecision) and effects of increasing total PUFA on depression risk are unclear as the evidence is of very low quality (downgraded once each for risk of bias, indirectness and inconsistency, Supplementary Tables 6 and 7).
Effects of increasing omega-3, omega-6 or total PUFA on depression severity and remission in those with existing depression
A single small trial assessed effects of LCn3 for 6 months in poor Iranian men with mild or moderate depression at baselineReference Tajalizadekhoob, Sharifi, Fakhrzadeh, Mirarefin, Ghaderpanahi and Badamchizade28, and found that Geriatric Depression Scale-15 score fell by >10% of baseline (suggesting reduced depression severity) in the higher versus lower LCn3 arm (mean difference −0.94, 95% CI −2.27 to 0.39, n = 61 participants). A further small study (n = 24) included participants with Parkinson's disease,Reference Pomponi, Loria, Salvati, Di Biase, Conte and Villella53 some of whom were depressed at baseline, and reported on remission, suggesting more remission in those on higher LCn3 (Supplementary Table 2). GRADE assessment suggests that effects of increasing LCn3 on risk of depression severity and risk of remission in those with existing depression are unclear as the evidence was of very low quality (depression severity downgraded twice for risk of bias, once for inconsistency; risk of remission absolute risk reduction 0.58, downgraded once for risk of bias and twice for indirectness, Supplementary Table 3). No trials of ALA, omega-6 or total PUFA included participants with depression at baseline.
Effects of increasing omega-3, omega-6 or total PUFA on risk of anxiety symptoms, severity and remission
Data were limited from trials of LCn3 assessing anxiety symptoms (Supplementary Tables 2 and 3). One study provided data on effects of LCn3 on risk of anxiety (RR 1.00, 95% CI 0.32–3.10); none provided data on remission. Five studies assessed effects of increasing LCn3 on anxiety symptoms, using four different scales (standardised mean difference 0.15, 95% CI 0.05–0.26, I 2 = 0%, n = 1378 participants, and no included studies were at low summary risk of bias, Fig. 3). No studies provided data on effects of ALA, omega-6 or total PUFA on anxiety incidence, remission or symptoms. GRADE assessment suggests that increasing LCn3 probably has little or no effect on anxiety symptoms (moderate-quality evidence, downgraded once for risk of bias, Supplementary Table 3).
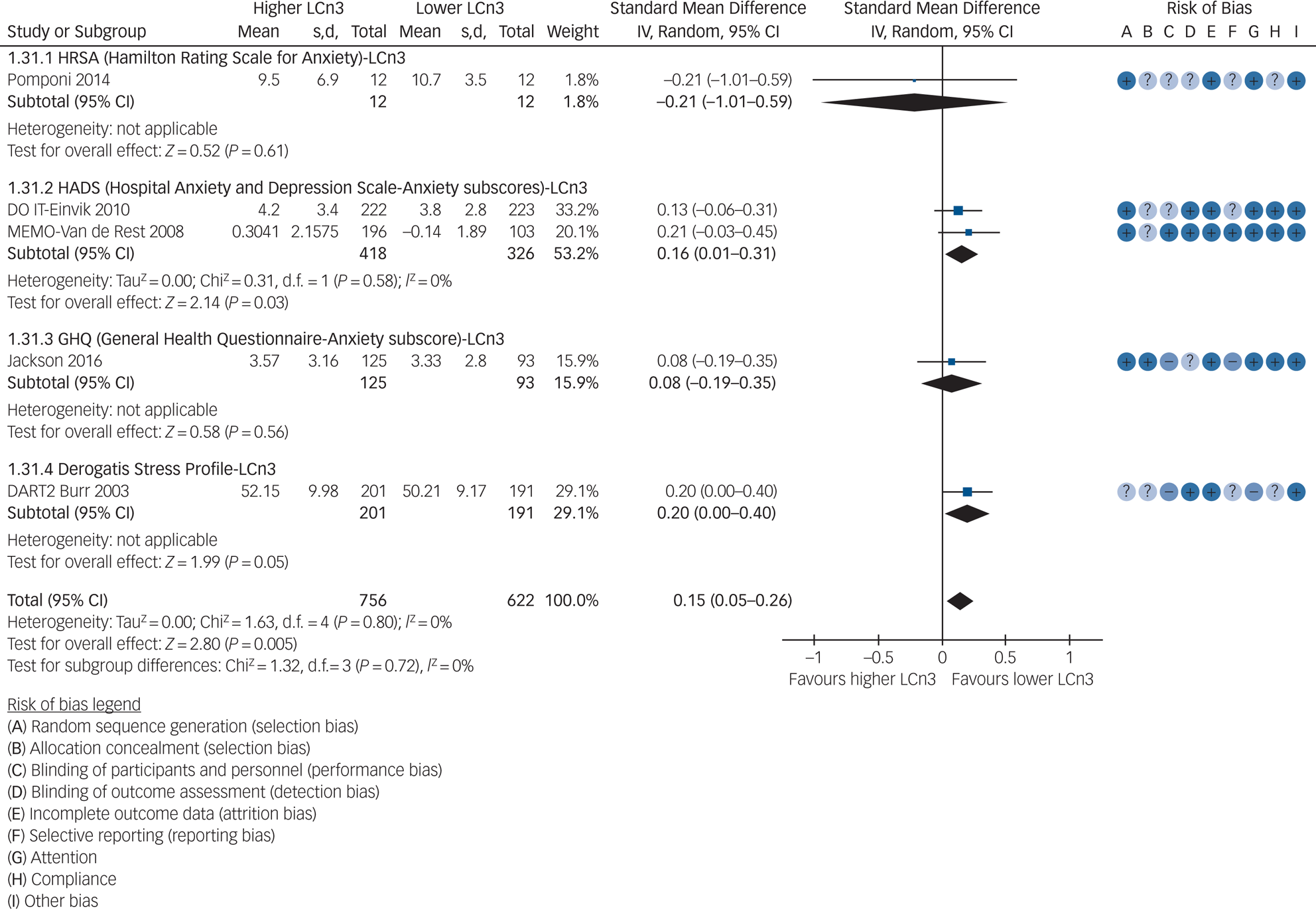
Fig. 3 Meta-analysis of trials randomising to higher versus lower long-chain omega-3 (LCn3) intake and assessing anxiety.
Secondary outcomes
Data on secondary outcomes are reported in Supplementary Text 1 and Supplementary Tables 8 and 9. Data were found on quality of life, carer stress, suicidality, adverse events, drop-outs and drop-outs owing to adverse events, but data were sparse, often poorly reported and may suffer from reporting bias. We did not identify any clear harms or benefits of interventions for these outcomes. We have formally systematically reviewed effects of omega-3, omega-6 and total PUFA on cancer, diabetes, cognition, inflammatory bowel disease, cardiovascular disease, functional outcomes, mortality, adiposity and lipids in sister reviews, so these outcomes are not reported here.Reference Abdelhamid, Brown, Brainard, Biswas, Thorpe and Moore15–Reference Thorpe, Ajabnoor, Ahmed, Abdelhamid and Hooper23
Discussion
GRADE assessment of our meta-analytic data suggests that increasing LCn3 probably has little or no effect on risk of depression or anxiety symptoms in those without depression or anxiety at baseline (moderate-quality evidence), but effects on depression severity and risk of remission in depression were unclear. Increasing ALA may increase risk of depression symptoms very slightly (1000 people would need to increase their ALA intake for one additional person to develop depression symptoms). Data on other outcomes and effects of increasing omega-6 and total PUFA were missing or of very low quality.
Strengths and limitations
Strengths of this review include our very broad search of long-term trials that assessed effects of omega-3, omega-6 or total PUFA on any outcomes,Reference Hooper, Abdelhamid, Brainard, Deane and Song22 and contact with many trial authors enabling us to include previously unpublished data. Evidence for the lack of impact of LCn3 on risk of depression symptoms comes from a broad range of trials, across thousands of men and women with diverse health status and depression risk, including large, long-term trials with low summary risk of bias. The broad set of trials also allowed thorough assessment of publication bias. We have used subgrouping to assess potential effects of LCn3, EPA and DHA dose, study duration (much of our data came from large trials of ≥3 years duration) and replacement of other nutrients (including omega-6, monounsaturated and saturated fats) on depression symptoms. Increasing LCn3, EPA or DHA dose, trial duration or altering nutrients replaced by LCn3 does not improve effectiveness of LCn3 on risk of depression symptoms.
Limitations include lack of information within trials on baseline LCn3 intake. Baseline intake of LCn3 could alter effectiveness of LCn3 supplementation, as increasing LCn3 would be more likely to be effective in those with poor baseline intakes. However, where trials reported baseline LCn3 intake or status, they did so in ways that are not comparable across trials (e.g. oily fish intake, erythrocyte membrane EPA, plasma LCn3), so we were unable to assess effects by baseline LCn3 status or intake. Although available data did not allow us to assess effects by omega-3/omega-6 ratio there was no suggestion of greater effects when omega-3 replaced omega-6, downplaying the importance of this ratio in depression and anxiety. The variety of methods of assessment of depression and anxiety symptoms, and limited clinical diagnoses of depression or anxiety (relying on scales of symptoms) may also limit clinical interpretation. However, these are the best data available on prevention of depression and anxiety, there are no previous systematic reviews of prevention and our collation of a broad database of all long-term trials of omega-3, omega-6 and total PUFA has allowed assessment of effects that are otherwise unpublished and inaccessible.Reference Hooper, Abdelhamid, Brainard, Deane and Song22 We carried out standardised mean difference analysis and reported effects in the single, most common scale.
Comparison with other research
The Multi-country Project on the Role of Diet, Food-related Behavior, and Obesity in the Prevention of Depression trial randomised participants to 1.4 g/d LCn3 plus additional micronutrients or placebo and found no effect on diagnosis of major depressive disorder after 1 year in 1025 overweight adults with subsyndromal depressive symptoms.Reference Bot, Brouwer, Roca, Kohls, Penninx and Watkins63 This trial is not included in our systematic review as the intervention was multifactorial (effects of LCn3 cannot be separated out), but confirms our review findings that LCn3 supplementation does not help to prevent depression. We found no previous systematic reviews of RCTs on effects of omega-6 or total PUFA, none separated out effects of ALA, and none assessed effects on prevention of depression. Systematic reviews on anxiety have included trials of very short duration and without controls.Reference Su, Tseng, Lin, Okubo, Chen and Chen64
Given that humans require at least 6 months to equilibrate fatty acids throughout our bodies when changes to LCn3 intake occur,Reference Browning, Walker, Mander, West, Madden and Gambell24 we were surprised to find only two small trials of LCn3 with a duration of at least 24 weeks that included participants with depression at baseline, to enable assessment of effects on depression severity and remission. As depression and anxiety are commonly recurring illnesses, longer-term health effects are crucial to understand, and we assumed we would find trials of polyunsaturated fats alongside effective antidepressants or anxiolytics compared with placebo and the same effective antidepressant or anxiolytic. None of our included trials clearly assessed dietary fats in combination with medications for depression or anxiety, which could potentiate effectiveness.
Shorter-term trials of omega-3 fats have been extensively reviewed. For example, a previous high-quality Cochrane systematic review of shorter trials of LCn3 in people with depression suggested small to modest non-clinically beneficial effects but queried risk of bias and publication bias in this data-set.Reference Appleton, Sallis, Perry, Ness and Churchill14 However, another systematic review of trials in major depression suggested efficacy at higher EPA doses and alongside antidepressants.Reference Mocking, Harmsen, Assies, Koeter, Ruhe and Schene65 Like the Cochrane review, which also used GRADE assessment,Reference Appleton, Sallis, Perry, Ness and Churchill14 we found that evidence of effects of LCn3 on depression severity and remission were of very low quality. Other recent systematic reviews of effects of omega-3 in people with existing depression have concluded that there were ‘mixed findings’ in older adults, suggesting that more high-quality, large-scale RCTs are needed,Reference Bai, Bo, Wu, Gai and Chi66 in call for trials in people with diagnosed depression and of longer duration,Reference Hallahan, Ryan, Hibbeln, Murray, Glynn and Ramsden67 and with a suggestion that combined EPA and DHA are of (non-significant) benefit in women (based on fewer than 400 participants).Reference Yang, Han, Qiao, Tian, Qi and Qiu68
LCn3 was mainly provided in supplementary form, so although there was no suggestion of different effects in trials of dietary advice or where oily fish was provided to participants compared with trials of LCn3 supplements, effects of dietary fish may differ (as dietary fish replaces other foods, and includes a wide range of additional nutrients including protein, selenium, iodine, calcium and magnesium).
Although LCn3 and ALA may protect against depression and anxiety in select individuals owing to specific genetic, dietary and/or metabolic characteristics, LCn3 and ALA will be harmful in other selected individuals. This systematic review suggests that any such benefits and harms are balanced, and that there will be no overall benefits on depression and anxiety symptoms of increasing LCn3 in general populations.
Implications for practice
Many adults take omega-3 supplements to improve their mental health. Our comprehensive systematic review and meta-analysis suggested that taking LCn3 supplements probably has little or no effect on risk of depression or anxiety symptoms (moderate-quality evidence). Results did not differ by risk of bias, omega-3 dose, duration or nutrients replaced. Effects on depression severity and remission were unclear (very-low-quality evidence). Physicians should not recommend omega-3 supplements for reducing depression or anxiety risk, and evidence of effectiveness in existing depression is of very low quality.
Research implications
Further methodologically strong, long-term trials (that focus on robust randomisation, allocation concealment, and blinding of participants, trial staff and outcome assessors, as well as adequately checking adherence in both the intervention and control arms) are needed to drive practice in people with existing depression and anxiety.
Supplementary material
Supplementary material is available online at https://doi.org/10.1192/bjp.2019.234.
Funding
The World Health Organization Nutrition Guidance Expert Advisory Group (NUGAG) Subgroup on Diet and Health (via the University of East Anglia) funded the research (grant number 2017/695622-0 to L.H., K.H.O.D., A.S.A., O.F.J., P.B. and S.H.). The funder had no role in data collection, data analysis, data interpretation or writing of the report. The funders were involved in study design during commissioning, and GRADE assessment was drafted by L.H., then discussed and agreed with NUGAG as part of guidance development. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis, as well as the decision to submit for publication.
Acknowledgements
The review authors thank all of the authors of primary studies who kindly provided us with the best set of data available, including: D. Kromhout, Wageningen University;Reference Kromhout, Giltay and Geleijnse30 Emily Chew, National Institutes of Health;Reference Chew, Clemons, Agron, Launer, Grodstein and Bernstein31 M.L. Burr, University of Wales and A. Ness, University of Bristol;Reference Burr, Ashfield-Watt, Dunstan, Fehily, Breay and Ashton33 G. Derosa and P. Maffioli, University of Pavia;Reference Derosa, Cicero, D'Angelo, Borghi and Maffioli34 S. Tokudome, National Institute of Health and Nutrition, Japan;Reference Tokudome, Kuriki, Yokoyama, Sasaki, Joh and Kamiya35 G. Einvik, Akershus University Hospital and H. Arnesen, Oslo University Hospital;Reference Einvik, Ekeberg, Lavik, Ellingsen, Klemsdal and Hjerkinn36 V. Danthiir, Commonwealth Scientific and Industrial Research Organisation Human Nutrition, Adelaide;Reference Danthiir, Hosking, Burns, Wilson, Nettelbeck and Calvaresi38 M. Hashimoto, Shimane University;Reference Hashimoto, Kato, Tanabe, Katakura, Mamun and Ohno40 P. Jackson, Northumbria University;Reference Jackson, Forster, Bell, Dick, Younger and Kennedy41 S. Schneider, Institut für Herzinfarktforschung, Germany;Reference Rauch, Schiele, Schneider, Diller, Victor and Gohlke50 Y. Freund-Levi, Karolinska Institutet;Reference Freund-Levi, Basun, Cederholm, Faxen-Irving, Garlind and Grut49 A. Dangour, London School of Hygiene & Tropical Medicine;Reference Dangour, Allen, Elbourne, Fasey, Fletcher and Hardy51 N. Parletta, University of South Australia;Reference Sinn, Milte, Street, Buckley, Coates and Petkov55 P. Galan, Université Paris;Reference Galan, Kesse-Guyot, Czernichow, Briancon, Blacher and Hercberg56 K. Tuttle, Sacred Heart Medical Center, Spokane.Reference Tuttle, Shuler, Packard, Milton, Daratha and Bibus57 Thanks also to the authors who replied but were not able to provide further details or confirmed no relevant outcomes, including A Sanyal, Virginia Commonwealth University, USA.Reference Sanyal, Abdelmalek, Suzuki, Cummings, Chojkier and Group37
The manuscript's guarantor affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. The guarantor attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.







eLetters
No eLetters have been published for this article.