Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood
- PMID: 10432279
- PMCID: PMC2195579
- DOI: 10.1084/jem.190.2.157
Telomere fluorescence measurements in granulocytes and T lymphocyte subsets point to a high turnover of hematopoietic stem cells and memory T cells in early childhood
Abstract
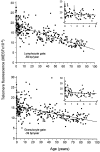
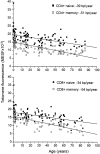
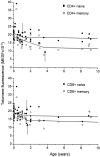
To study telomere length dynamics in hematopoietic cells with age, we analyzed the average length of telomere repeat sequences in diverse populations of nucleated blood cells. More than 500 individuals ranging in age from 0 to 90 yr, including 36 pairs of monozygous and dizygotic twins, were analyzed using quantitative fluorescence in situ hybridization and flow cytometry. Granulocytes and naive T cells showed a parallel biphasic decline in telomere length with age that most likely reflected accumulated cell divisions in the common precursors of both cell types: hematopoietic stem cells. Telomere loss was very rapid in the first year, and continued for more than eight decades at a 30-fold lower rate. Memory T cells also showed an initial rapid decline in telomere length with age. However, in contrast to naive T cells, this decline continued for several years, and in older individuals lymphocytes typically had shorter telomeres than did granulocytes. Our findings point to a dramatic decline in stem cell turnover in early childhood and support the notion that cell divisions in hematopoietic stem cells and T cells result in loss of telomeric DNA.
Figures

Similar articles
-
Synchrony of telomere length among hematopoietic cells.Exp Hematol. 2010 Oct;38(10):854-9. doi: 10.1016/j.exphem.2010.06.010. Epub 2010 Jun 25. Exp Hematol. 2010. PMID: 20600576 Free PMC article.
-
Longitudinal studies of telomere length in feline blood cells: implications for hematopoietic stem cell turnover in vivo.Exp Hematol. 2002 Oct;30(10):1147-52. doi: 10.1016/s0301-472x(02)00888-3. Exp Hematol. 2002. PMID: 12384145
-
Limited telomere shortening in hematopoietic stem cells after transplantation.Ann N Y Acad Sci. 2001 Jun;938:1-7; discussion 7-8. doi: 10.1111/j.1749-6632.2001.tb03568.x. Ann N Y Acad Sci. 2001. PMID: 11458496
-
Different rates of telomere attrition in peripheral lymphocytes in a pair of dizygotic twins with hematopoietic chimerism.Aging Cell. 2008 Oct;7(5):663-6. doi: 10.1111/j.1474-9726.2008.00413.x. Epub 2008 Jul 24. Aging Cell. 2008. PMID: 18616638 Review.
-
Telomere length, aging, and somatic cell turnover.J Exp Med. 1999 Jul 19;190(2):153-6. doi: 10.1084/jem.190.2.153. J Exp Med. 1999. PMID: 10432278 Free PMC article. Review. No abstract available.
Cited by
-
Comprehensive snapshots of natural killer cells functions, signaling, molecular mechanisms and clinical utilization.Signal Transduct _target Ther. 2024 Nov 8;9(1):302. doi: 10.1038/s41392-024-02005-w. Signal Transduct _target Ther. 2024. PMID: 39511139 Free PMC article. Review.
-
Telomere Length and Telomerase Activity as Potential Biomarkers for Gastrointestinal Cancer.Cancers (Basel). 2024 Oct 1;16(19):3370. doi: 10.3390/cancers16193370. Cancers (Basel). 2024. PMID: 39409990 Free PMC article. Review.
-
A Self-Activating IL-15 Chimeric Cytokine Receptor to Empower Cancer Immunotherapy.Immuno_targets Ther. 2024 Oct 10;13:513-524. doi: 10.2147/ITT.S490498. eCollection 2024. Immuno_targets Ther. 2024. PMID: 39403195 Free PMC article.
-
Inherited Telomere Biology Disorders: Pathophysiology, Clinical Presentation, Diagnostics, and Treatment.Transfus Med Hemother. 2024 Jul 30;51(5):292-309. doi: 10.1159/000540109. eCollection 2024 Oct. Transfus Med Hemother. 2024. PMID: 39371255 Free PMC article. Review.
-
Current and Future Trends of Colorectal Cancer Treatment: Exploring Advances in Immunotherapy.Cancers (Basel). 2024 May 24;16(11):1995. doi: 10.3390/cancers16111995. Cancers (Basel). 2024. PMID: 38893120 Free PMC article. Review.
References
-
- Lansdorp P.M. Self-renewal of stem cells. Biol. Blood Marrow Transplant. 1997;3:171–178. - PubMed
-
- Wynn R.F., Cross M.A., Hatton C., Will A.M., Lashford L.S., Dexter T.M., Testa N.G. Accelerated telomere shortening in young recipients of allogeneic bone-marrow transplants. Lancet. 1998;351:178–181. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

