A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta
- PMID: 11371646
- PMCID: PMC33469
- DOI: 10.1073/pnas.101133498
A rescue factor abolishing neuronal cell death by a wide spectrum of familial Alzheimer's disease genes and Abeta
Erratum in
- Proc Natl Acad Sci U S A 2001 Oct 23;98(22):12854
Abstract
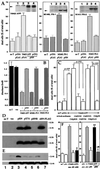
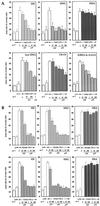
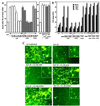
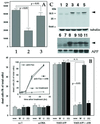
Through functional expression screening, we identified a gene, designated Humanin (HN) cDNA, which encodes a short polypeptide and abolishes death of neuronal cells caused by multiple different types of familial Alzheimer's disease genes and by Abeta amyloid, without effect on death by Q79 or superoxide dismutase-1 mutants. Transfected HN cDNA was transcribed to the corresponding polypeptide and then was secreted into the cultured medium. The rescue action clearly depended on the primary structure of HN. This polypeptide would serve as a molecular clue for the development of new therapeutics for Alzheimer's disease _targeting neuroprotection.
Figures

Similar articles
-
Mechanisms of neuroprotection by a novel rescue factor humanin from Swedish mutant amyloid precursor protein.Biochem Biophys Res Commun. 2001 May 4;283(2):460-8. doi: 10.1006/bbrc.2001.4765. Biochem Biophys Res Commun. 2001. PMID: 11327724
-
Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults.J Neurosci. 2001 Dec 1;21(23):9235-45. doi: 10.1523/JNEUROSCI.21-23-09235.2001. J Neurosci. 2001. PMID: 11717357 Free PMC article.
-
["Death and survival of neuronal cells exposed to Alzheimer's disease-relevant insults"].Nihon Yakurigaku Zasshi. 2002 Nov;120(1):11P-15P. Nihon Yakurigaku Zasshi. 2002. PMID: 12491768 Review. Japanese.
-
[Neuronal cell death by Alzheimer's disease-relevant insults and its rescue].Nihon Ronen Igakkai Zasshi. 2003 Jan;40(1):36-40. doi: 10.3143/geriatrics.40.36. Nihon Ronen Igakkai Zasshi. 2003. PMID: 12649845 Japanese.
-
Death and survival of neuronal cells exposed to Alzheimer's insults.J Neurosci Res. 2002 Nov 1;70(3):380-91. doi: 10.1002/jnr.10354. J Neurosci Res. 2002. PMID: 12391601 Review.
Cited by
-
Deep proteome coverage based on ribosome profiling aids mass spectrometry-based protein and peptide discovery and provides evidence of alternative translation products and near-cognate translation initiation events.Mol Cell Proteomics. 2013 Jul;12(7):1780-90. doi: 10.1074/mcp.M113.027540. Epub 2013 Feb 21. Mol Cell Proteomics. 2013. PMID: 23429522 Free PMC article.
-
Mitochondrial function in development and disease.Dis Model Mech. 2021 Jun 1;14(6):dmm048912. doi: 10.1242/dmm.048912. Epub 2021 Jun 11. Dis Model Mech. 2021. PMID: 34114603 Free PMC article. Review.
-
Mitochondrial-derived peptides: Antidiabetic functions and evolutionary perspectives.Peptides. 2024 Feb;172:171147. doi: 10.1016/j.peptides.2023.171147. Epub 2023 Dec 29. Peptides. 2024. PMID: 38160808 Free PMC article. Review.
-
Humanin attenuates apoptosis induced by DRPLA proteins with expanded polyglutamine stretches.J Mol Neurosci. 2005;25(2):165-9. doi: 10.1385/JMN:25:2:165. J Mol Neurosci. 2005. PMID: 15784964
-
The cardiac-enriched microprotein mitolamban regulates mitochondrial respiratory complex assembly and function in mice.Proc Natl Acad Sci U S A. 2022 Feb 8;119(6):e2120476119. doi: 10.1073/pnas.2120476119. Proc Natl Acad Sci U S A. 2022. PMID: 35101990 Free PMC article.
References
-
- Shastry B S, Giblin F J. Brain Res Bull. 1999;48:121–127. - PubMed
-
- Yamatsuji T, Okamoto T, Takeda S, Fukumoto H, Iwatsubo T, Suzuki N, Asami-Odaka A, Ireland S, Kinane T B, Nishimoto I. Science. 1996;272:1349–1352. - PubMed
-
- Zhao B, Chrest F J, Horton W E, Jr, Sisodia S S, Kusiak J W. J Neurosci Res. 1997;47:253–263. - PubMed
-
- Luo J J, Wallace W, Riccioni T, Ingram D K, Roth G S, Kusiak J W. J Neurosci Res. 1999;55:629–642. - PubMed
-
- Wolozin B, Iwasaki K, Vito P, Ganjei J K, Lacana E, Sunderland T, Zhao B, Kusiak J W, Wasco W, D'Adamio L. Science. 1996;274:1710–1713. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

