Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification
- PMID: 12060695
- PMCID: PMC117299
- DOI: 10.1093/nar/gnf056
Relative quantification of 40 nucleic acid sequences by multiplex ligation-dependent probe amplification
Abstract
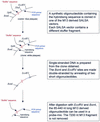
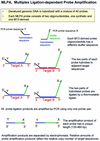
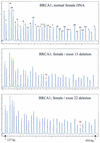
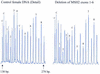
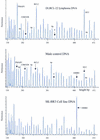
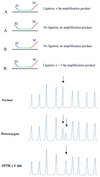
We describe a new method for relative quantification of 40 different DNA sequences in an easy to perform reaction requiring only 20 ng of human DNA. Applications shown of this multiplex ligation-dependent probe amplification (MLPA) technique include the detection of exon deletions and duplications in the human BRCA1, MSH2 and MLH1 genes, detection of trisomies such as Down's syndrome, characterisation of chromosomal aberrations in cell lines and tumour samples and SNP/mutation detection. Relative quantification of mRNAs by MLPA will be described elsewhere. In MLPA, not sample nucleic acids but probes added to the samples are amplified and quantified. Amplification of probes by PCR depends on the presence of probe _target sequences in the sample. Each probe consists of two oligonucleotides, one synthetic and one M13 derived, that hybridise to adjacent sites of the _target sequence. Such hybridised probe oligonucleotides are ligated, permitting subsequent amplification. All ligated probes have identical end sequences, permitting simultaneous PCR amplification using only one primer pair. Each probe gives rise to an amplification product of unique size between 130 and 480 bp. Probe _target sequences are small (50-70 nt). The prerequisite of a ligation reaction provides the opportunity to discriminate single nucleotide differences.
Figures


Similar articles
-
Identification and characterization of genomic rearrangements of MSH2 and MLH1 in Lynch syndrome (HNPCC) by novel techniques.Hum Mutat. 2003 Sep;22(3):258. doi: 10.1002/humu.9171. Hum Mutat. 2003. PMID: 12938096
-
Genomic deletions in MSH2 or MLH1 are a frequent cause of hereditary non-polyposis colorectal cancer: identification of novel and recurrent deletions by MLPA.Hum Mutat. 2003 Dec;22(6):428-33. doi: 10.1002/humu.10291. Hum Mutat. 2003. PMID: 14635101
-
Confirmation of single exon deletions in MLH1 and MSH2 using quantitative polymerase chain reaction.J Mol Diagn. 2008 Jul;10(4):355-60. doi: 10.2353/jmoldx.2008.080021. Epub 2008 Jun 13. J Mol Diagn. 2008. PMID: 18556772 Free PMC article.
-
A sense of closeness: protein detection by proximity ligation.Curr Opin Biotechnol. 2003 Feb;14(1):82-6. doi: 10.1016/s0958-1669(02)00011-3. Curr Opin Biotechnol. 2003. PMID: 12566006 Review.
-
Proximity ligation assay: an ultrasensitive method for protein quantification and its applications in pathogen detection.Appl Microbiol Biotechnol. 2021 Feb;105(3):923-935. doi: 10.1007/s00253-020-11049-1. Epub 2021 Jan 11. Appl Microbiol Biotechnol. 2021. PMID: 33427935 Review.
Cited by
-
Cytokine expression patterns in hospitalized children with Bordetella pertussis, Rhinovirus or co-infection.Sci Rep. 2021 May 26;11(1):10948. doi: 10.1038/s41598-021-89538-0. Sci Rep. 2021. PMID: 34040002 Free PMC article.
-
Simultaneous detection of BRCA mutations and large genomic rearrangements in germline DNA and FFPE tumor samples.Onco_target. 2016 Sep 20;7(38):61845-61859. doi: 10.18632/onco_target.11259. Onco_target. 2016. PMID: 27533253 Free PMC article.
-
A Robust Protocol for Using Multiplexed Droplet Digital PCR to Quantify Somatic Copy Number Alterations in Clinical Tissue Specimens.PLoS One. 2016 Aug 18;11(8):e0161274. doi: 10.1371/journal.pone.0161274. eCollection 2016. PLoS One. 2016. PMID: 27537682 Free PMC article.
-
Copy Number Variations in Pancreatic Cancer: From Biological Significance to Clinical Utility.Int J Mol Sci. 2023 Dec 27;25(1):391. doi: 10.3390/ijms25010391. Int J Mol Sci. 2023. PMID: 38203561 Free PMC article. Review.
-
Molecular characterization of head and neck cancer: how close to personalized _targeted therapy?Mol Diagn Ther. 2012 Aug 1;16(4):209-22. doi: 10.2165/11635330-000000000-00000. Mol Diagn Ther. 2012. PMID: 22873739 Free PMC article.
References
-
- Petrij-Bosch A., Peelen,T., van Vliet,M., van Eijk,R., Olmer,R., Drusedau,M., Hogervorst,F.B., Hageman,S., Arts,P.J., Ligtenberg,M.J. et al. (1997) BRCAI genomic deletions are major founder mutations in Dutch breast cancer patients. Nature Genet., 17, 341–345. - PubMed
-
- Wijnen J., van der Klift,H., Vasen,H., Khan,P.M., Menko,F., Tops,C., Meijers Heijboer,H., Lindhout,D., Moller,P. and Fodde,R. (1998) MSH2 genomic deletions are a frequent cause of HNPCC. Nature Genet., 20, 326–328. - PubMed
-
- Kauraniemi P., Barlund,M., Monni,O. and Kallioniemi,A. (2001) New amplified and highly expressed genes discovered in the ERBB2 amplicon in breast cancer by cDNA microarrays. Cancer Res., 61, 8235–8240. - PubMed
-
- Leyland-Jones B. and Smith,I. (2001) Role of herceptin in primary breast cancer. Oncology, 61 (Suppl. 2), 83–91. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

