Activation of the Wnt signaling pathway in chronic lymphocytic leukemia
- PMID: 14973184
- PMCID: PMC365753
- DOI: 10.1073/pnas.0308648100
Activation of the Wnt signaling pathway in chronic lymphocytic leukemia
Abstract
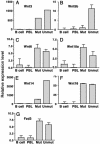
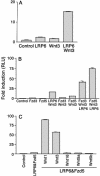
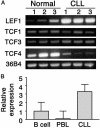
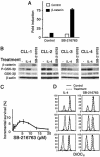
B cell chronic lymphocytic leukemia (CLL) is characterized by an accumulation of mature, functionally incompetent B cells. Wnts are a large family of secreted glycoproteins involved in cell proliferation, differentiation, and oncogenesis. The classical Wnt signaling cascade inhibits the activity of the enzyme glycogen synthase kinase-3beta, augmenting beta-catenin translocation to the nucleus, and the transcription of _target genes. Little is known about the potential roles of Wnt signaling in CLL. In this study, we quantified the gene expression profiles of the Wnt family, and their cognate frizzled (Fzd) receptors in primary CLL cells, and determined the role of Wnt signaling in promoting CLL cell survival. Wnt3, Wnt5b, Wnt6, Wnt10a, Wnt14, and Wnt16, as well as the Wnt receptor Fzd3, were highly expressed in CLL, compared with normal B cells. Three lines of evidence suggested that the Wnt signaling pathway was active in CLL. First, the Wnt/beta-catenin-regulated transcription factor lymphoid-enhancing factor-1, and its downstream _target cyclin D1, were overexpressed in CLL. Second, a pharmacological inhibitor of glycogen synthase kinase-3 beta, SB-216763, activated beta-catenin-mediated transcription, and enhanced the survival of CLL lymphocytes. Third, Wnt/beta-catenin signaling was diminished by an analog of a nonsteroidal antiinflammatory drug (R-etodolac), at concentrations that increased apoptosis of CLL cells. Taken together, these results indicate that Wnt signaling genes are overexpressed and are active in CLL. Uncontrolled Wnt signaling may contribute to the defect in apoptosis that characterizes this malignancy.
Figures
Similar articles
-
Identification of a Wnt/beta-catenin signaling pathway in human thyroid cells.Endocrinology. 2001 Dec;142(12):5261-6. doi: 10.1210/endo.142.12.8554. Endocrinology. 2001. PMID: 11713224
-
Alterations of beta-catenin and Tcf-4 instead of GSK-3beta contribute to activation of Wnt pathway in hepatocellular carcinoma.Chin Med J (Engl). 2003 Dec;116(12):1885-92. Chin Med J (Engl). 2003. PMID: 14687479
-
The human Frizzled 6 (HFz6) acts as a negative regulator of the canonical Wnt. beta-catenin signaling cascade.J Biol Chem. 2004 Apr 9;279(15):14879-88. doi: 10.1074/jbc.M306421200. Epub 2004 Jan 27. J Biol Chem. 2004. PMID: 14747478
-
Wnt/β-catenin/LEF-1 signaling in chronic lymphocytic leukemia (CLL): a _target for current and potential therapeutic options.Curr Cancer Drug _targets. 2010 Nov;10(7):716-27. doi: 10.2174/156800910793605794. Curr Cancer Drug _targets. 2010. PMID: 20578984 Review.
-
Caught up in a Wnt storm: Wnt signaling in cancer.Biochim Biophys Acta. 2003 Jun 5;1653(1):1-24. doi: 10.1016/s0304-419x(03)00005-2. Biochim Biophys Acta. 2003. PMID: 12781368 Review.
Cited by
-
Digital gene expression tag profiling analysis of the gene expression patterns regulating the early stage of mouse spermatogenesis.PLoS One. 2013;8(3):e58680. doi: 10.1371/journal.pone.0058680. Epub 2013 Mar 15. PLoS One. 2013. PMID: 23554914 Free PMC article.
-
Absence of sclerostin adversely affects B-cell survival.J Bone Miner Res. 2012 Jul;27(7):1451-61. doi: 10.1002/jbmr.1608. J Bone Miner Res. 2012. PMID: 22434688 Free PMC article.
-
The role of sirtuin 2 activation by nicotinamide phosphoribosyltransferase in the aberrant proliferation and survival of myeloid leukemia cells.Haematologica. 2012 Apr;97(4):551-9. doi: 10.3324/haematol.2011.055236. Epub 2011 Dec 29. Haematologica. 2012. PMID: 22207684 Free PMC article.
-
To β or Not to β: How Important Is β-Catenin Dependent and Independent WNT Signaling in CLL?Cancers (Basel). 2022 Dec 28;15(1):194. doi: 10.3390/cancers15010194. Cancers (Basel). 2022. PMID: 36612190 Free PMC article. Review.
-
The functional roles of the circRNA/Wnt axis in cancer.Mol Cancer. 2022 May 5;21(1):108. doi: 10.1186/s12943-022-01582-0. Mol Cancer. 2022. PMID: 35513849 Free PMC article. Review.
References
-
- Kipps, T. J. (2000) Curr. Opin. Hematol. 7, 223-234. - PubMed
-
- Damle, R. N., Wasil, T., Fais, F., Ghiotto, F., Valetto, A., Allen, S. L., Buchbinder, A., Budman, D., Dittmar, K., Kolitz, J., et al. (1999) Blood 94, 1840-1847. - PubMed
-
- Hamblin, T. J., Davis, Z., Gardiner, A., Oscier, D. G. & Stevenson, F. K. (1999) Blood 94, 1848-1854. - PubMed
-
- Chen, L., Widhopf, G., Huynh, L., Rassenti, L., Rai, K. R., Weiss, A. & Kipps, T. J. (2002) Blood 100, 4609-4614. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

