Btk is required for an efficient response to erythropoietin and for SCF-controlled protection against TRAIL in erythroid progenitors
- PMID: 15007095
- PMCID: PMC2212722
- DOI: 10.1084/jem.20031109
Btk is required for an efficient response to erythropoietin and for SCF-controlled protection against TRAIL in erythroid progenitors
Erratum in
- J Exp Med. 2004 Apr 5;199(7):following 1031
Abstract
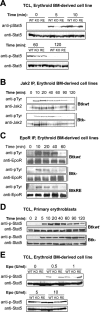
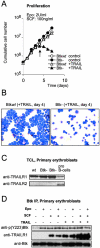
Regulation of survival, expansion, and differentiation of erythroid progenitors requires the well-controlled activity of signaling pathways induced by erythropoietin (Epo) and stem cell factor (SCF). In addition to qualitative regulation of signaling pathways, quantitative control may be essential to control appropriate cell numbers in peripheral blood. We demonstrate that Bruton's tyrosine kinase (Btk) is able to associate with the Epo receptor (EpoR) and Jak2, and is a substrate of Jak2. Deficiency of Btk results in reduced and delayed phosphorylation of the EpoR, Jak2, and downstream signaling molecules such as Stat5 and PLCgamma1 as well as in decreased responsiveness to Epo. As a result, expansion of erythroid progenitors lacking Btk is impaired at limiting concentrations of Epo and SCF. In addition, we show that SCF induces Btk to interact with TNF-related apoptosis-inducing ligand (TRAIL)-receptor 1 and that lack of Btk results in increased sensitivity to TRAIL-induced apoptosis. Together, our results indicate that Btk is a novel, quantitative regulator of Epo/SCF-dependent expansion and survival in erythropoiesis.
Figures


Similar articles
-
Control of erythropoiesis by erythropoietin and stem cell factor: a novel role for Bruton's tyrosine kinase.Cell Cycle. 2004 Jul;3(7):876-9. Epub 2004 Jul 2. Cell Cycle. 2004. PMID: 15254422 Review.
-
Lnk inhibits erythropoiesis and Epo-dependent JAK2 activation and downstream signaling pathways.Blood. 2005 Jun 15;105(12):4604-12. doi: 10.1182/blood-2004-10-4093. Epub 2005 Feb 10. Blood. 2005. PMID: 15705783 Free PMC article.
-
Co-operative signalling mechanisms required for erythroid precursor expansion in response to erythropoietin and stem cell factor.Br J Haematol. 2005 Jul;130(1):121-9. doi: 10.1111/j.1365-2141.2005.05580.x. Br J Haematol. 2005. PMID: 15982354
-
Erythropoietin and Friend virus gp55 activate different JAK/STAT pathways through the erythropoietin receptor in erythroid cells.Mol Cell Biol. 1998 Mar;18(3):1172-80. doi: 10.1128/MCB.18.3.1172. Mol Cell Biol. 1998. PMID: 9488432 Free PMC article.
-
STAT5 as a Key Protein of Erythropoietin Signalization.Int J Mol Sci. 2021 Jul 1;22(13):7109. doi: 10.3390/ijms22137109. Int J Mol Sci. 2021. PMID: 34281163 Free PMC article. Review.
Cited by
-
Antiproliferative and Apoptotic Effect of Curcumin and TRAIL (TNF Related Apoptosis inducing Ligand) in Chronic Myeloid Leukaemic Cells.J Clin Diagn Res. 2016 Apr;10(4):XC01-XC05. doi: 10.7860/JCDR/2016/18507.7579. Epub 2016 Apr 1. J Clin Diagn Res. 2016. PMID: 27190933 Free PMC article.
-
Differential regulation of Foxo3a _target genes in erythropoiesis.Mol Cell Biol. 2007 May;27(10):3839-3854. doi: 10.1128/MCB.01662-06. Mol Cell Biol. 2007. PMID: 17353275 Free PMC article.
-
Erythropoietin Intensifies the Proapoptotic Activity of LFM-A13 in Cells and in a Mouse Model of Colorectal Cancer.Int J Mol Sci. 2018 Apr 23;19(4):1262. doi: 10.3390/ijms19041262. Int J Mol Sci. 2018. PMID: 29690619 Free PMC article.
-
Dynamics of Chromatin Accessibility During Hematopoietic Stem Cell Differentiation Into Progressively Lineage-Committed Progeny.Stem Cells. 2023 May 15;41(5):520-539. doi: 10.1093/stmcls/sxad022. Stem Cells. 2023. PMID: 36945732 Free PMC article.
-
Ibrutinib inhibits SDF1/CXCR4 mediated migration in AML.Onco_target. 2014 Oct 30;5(20):9930-8. doi: 10.18632/onco_target.2479. Onco_target. 2014. PMID: 25294819 Free PMC article.
References
-
- Wu, H., X. Liu, R. Jaenisch, and H.F. Lodish. 1995. Generation of committed erythroid BFU-E and CFU-E progenitors does not require erythropoietin or the erythropoietin receptor. Cell. 83:59–67. - PubMed
-
- Flanagan, J.G., D.C. Chan, and P. Leder. 1991. Transmembrane form of the kit ligand growth factor is determined by alternative splicing and is missing in the Sld mutant. Cell. 64:1025–1035. - PubMed
-
- Shetty, V., S. Hussaini, L. Broady-Robinson, K. Allampallam, S. Mundle, R. Borok, E. Broderick, L. Mazzoran, F. Zorat, and A. Raza. 2000. Intramedullary apoptosis of hematopoietic cells in myelodysplastic syndrome patients can be massive: apoptotic cells recovered from high-density fraction of bone marrow aspirates. Blood. 96:1388–1392. - PubMed
-
- De Maria, R., A. Zeuner, A. Eramo, C. Domenichelli, D. Bonci, F. Grignani, S.M. Srinivasula, E.S. Alnemri, U. Testa, and C. Peschle. 1999. Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1. Nature. 401:489–493. - PubMed
-
- Dai, C.H., J.O. Price, T. Brunner, and S.B. Krantz. 1998. Fas ligand is present in human erythroid colony-forming cells and interacts with Fas induced by interferon gamma to produce erythroid cell apoptosis. Blood. 91:1235–1242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

