A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change
- PMID: 16339891
- PMCID: PMC1367327
- DOI: 10.1529/biophysj.105.073536
A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change
Abstract
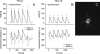
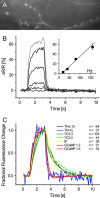
Genetically encoded calcium biosensors have become valuable tools in cell biology and neuroscience, but some aspects such as signal strength and response kinetics still need improvement. Here we report the generation of a FRET-based calcium biosensor employing troponin C as calcium-binding moiety that is fast, is stable in imaging experiments, and shows a significantly enhanced fluorescence change. These improvements were achieved by engineering magnesium and calcium-binding properties within the C-terminal lobe of troponin C and by the incorporation of circularly permuted variants of the green fluorescent protein. This sensor named TN-XL shows a maximum fractional fluorescence change of 400% in its emission ratio and linear response properties over an expanded calcium regime. When imaged in vivo at presynaptic motoneuron terminals of transgenic fruit flies, TN-XL exhibits highly reproducible fluorescence signals with the fastest rise and decay times of all calcium biosensors known so far.
Figures
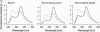

Similar articles
-
Live-cell transforms between Ca2+ transients and FRET responses for a troponin-C-based Ca2+ sensor.Biophys J. 2007 Dec 1;93(11):4031-40. doi: 10.1529/biophysj.107.109629. Epub 2007 Aug 17. Biophys J. 2007. PMID: 17704158 Free PMC article.
-
Confocal FLIM of genetically encoded FRET sensors for quantitative Ca2+ imaging.Cold Spring Harb Protoc. 2014 Dec 1;2014(12):1328-32. doi: 10.1101/pdb.prot077040. Cold Spring Harb Protoc. 2014. PMID: 25447281
-
In vivo performance of genetically encoded indicators of neural activity in flies.J Neurosci. 2005 May 11;25(19):4766-78. doi: 10.1523/JNEUROSCI.4900-04.2005. J Neurosci. 2005. PMID: 15888652 Free PMC article.
-
Troponin C-based biosensors: a new family of genetically encoded indicators for in vivo calcium imaging in the nervous system.Cell Calcium. 2007 Oct-Nov;42(4-5):351-61. doi: 10.1016/j.ceca.2007.02.011. Epub 2007 Apr 23. Cell Calcium. 2007. PMID: 17451806 Review.
-
Protein biosensors based on the principle of fluorescence resonance energy transfer for monitoring cellular dynamics.Biotechnol Lett. 2006 Dec;28(24):1971-82. doi: 10.1007/s10529-006-9193-5. Epub 2006 Oct 5. Biotechnol Lett. 2006. PMID: 17021660 Review.
Cited by
-
Fluorescent Protein-Based Sensors for Detecting Essential Metal Ions across the Tree of Life.ACS Sens. 2024 Apr 26;9(4):1622-1643. doi: 10.1021/acssensors.3c02695. Epub 2024 Apr 8. ACS Sens. 2024. PMID: 38587931 Review.
-
Development of an Efficient FRET-Based Ratiometric Uranium Biosensor.Biosensors (Basel). 2023 May 19;13(5):561. doi: 10.3390/bios13050561. Biosensors (Basel). 2023. PMID: 37232922 Free PMC article.
-
A BRET Ca2+ sensor enables high-throughput screening in the presence of background fluorescence.Sci Signal. 2022 Aug 16;15(747):eabq7618. doi: 10.1126/scisignal.abq7618. Epub 2022 Aug 16. Sci Signal. 2022. PMID: 35973028 Free PMC article.
-
Resource for FRET-Based Biosensor Optimization.Front Cell Dev Biol. 2022 Jun 20;10:885394. doi: 10.3389/fcell.2022.885394. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35794864 Free PMC article. Review.
-
Fluorescent Indicators For Biological Imaging of Monatomic Ions.Front Cell Dev Biol. 2022 Apr 27;10:885440. doi: 10.3389/fcell.2022.885440. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35573682 Free PMC article. Review.
References
-
- Zhang, J., R. E. Campbell, A. Y. Ting, and R. Y. Tsien. 2002. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 3:906–918. - PubMed
-
- Miyawaki, A. 2003. Visualization of the spatial and temporal dynamics of intracellular signaling. Dev. Cell. 4:295–305. - PubMed
-
- Griesbeck, O. 2004. Fluorescent proteins as sensors for cellular functions. Curr. Opin. Neurobiol. 14:636–641. - PubMed
-
- Suzuki, H., R. Kerr, L. Bianchi, C. Frokjaer-Jensen, D. Slone, J. Xue, B. Gerstbrein, M. Driscoll, and W. R. Schafer. 2003. In Vivo imaging of C. elegans mechanosensory neurons demonstrates a specific role for the MEC-4 channel in the process of gentle touch sensation. Neuron. 39:1005–1017. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

