Neuromusculoskeletal modeling: estimation of muscle forces and joint moments and movements from measurements of neural command
- PMID: 16467928
- PMCID: PMC1357215
- DOI: 10.1123/jab.20.4.367
Neuromusculoskeletal modeling: estimation of muscle forces and joint moments and movements from measurements of neural command
Abstract
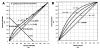
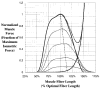
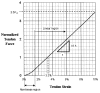
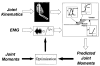
This paper provides an overview of forward dynamic neuromusculoskeletal modeling. The aim of such models is to estimate or predict muscle forces, joint moments, and/or joint kinematics from neural signals. This is a four-step process. In the first step, muscle activation dynamics govern the transformation from the neural signal to a measure of muscle activation-a time varying parameter between 0 and 1. In the second step, muscle contraction dynamics characterize how muscle activations are transformed into muscle forces. The third step requires a model of the musculoskeletal geometry to transform muscle forces to joint moments. Finally, the equations of motion allow joint moments to be transformed into joint movements. Each step involves complex nonlinear relationships. The focus of this paper is on the details involved in the first two steps, since these are the most challenging to the biomechanician. The global process is then explained through applications to the study of predicting isometric elbow moments and dynamic knee kinetics.
Figures
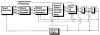

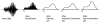


Similar articles
-
Estimation of muscle forces and joint moments using a forward-inverse dynamics model.Med Sci Sports Exerc. 2005 Nov;37(11):1911-6. doi: 10.1249/01.mss.0000176684.24008.6f. Med Sci Sports Exerc. 2005. PMID: 16286861
-
Prediction of joint moments using a neural network model of muscle activations from EMG signals.IEEE Trans Neural Syst Rehabil Eng. 2002 Mar;10(1):30-7. doi: 10.1109/TNSRE.2002.1021584. IEEE Trans Neural Syst Rehabil Eng. 2002. PMID: 12173737
-
Increasing level of neuromusculoskeletal model personalisation to investigate joint contact forces in cerebral palsy: A twin case study.Clin Biomech (Bristol). 2020 Feb;72:141-149. doi: 10.1016/j.clinbiomech.2019.12.011. Epub 2019 Dec 18. Clin Biomech (Bristol). 2020. PMID: 31877532
-
Feasibility of using EMG driven neuromusculoskeletal model for prediction of dynamic movement of the elbow.J Electromyogr Kinesiol. 2005 Feb;15(1):12-26. doi: 10.1016/j.jelekin.2004.06.007. J Electromyogr Kinesiol. 2005. PMID: 15642650
-
Model-based estimation of muscle forces exerted during movements.Clin Biomech (Bristol). 2007 Feb;22(2):131-54. doi: 10.1016/j.clinbiomech.2006.09.005. Epub 2006 Oct 27. Clin Biomech (Bristol). 2007. PMID: 17070969 Review.
Cited by
-
Lower Limb Joint Torque Prediction Using Long Short-Term Memory Network and Gaussian Process Regression.Sensors (Basel). 2023 Dec 2;23(23):9576. doi: 10.3390/s23239576. Sensors (Basel). 2023. PMID: 38067948 Free PMC article.
-
Muscle Synergies Facilitate Computational Prediction of Subject-Specific Walking Motions.Front Bioeng Biotechnol. 2016 Oct 13;4:77. doi: 10.3389/fbioe.2016.00077. eCollection 2016. Front Bioeng Biotechnol. 2016. PMID: 27790612 Free PMC article.
-
Patellofemoral contact forces after ACL reconstruction: A longitudinal study.J Biomech. 2022 Mar;134:110993. doi: 10.1016/j.jbiomech.2022.110993. Epub 2022 Feb 10. J Biomech. 2022. PMID: 35182902 Free PMC article.
-
How Many Muscles? Optimal Muscles Set Search for Optimizing Myocontrol Performance.Front Comput Neurosci. 2021 Oct 7;15:668579. doi: 10.3389/fncom.2021.668579. eCollection 2021. Front Comput Neurosci. 2021. PMID: 34690729 Free PMC article.
-
A Differentiable Dynamic Model for Musculoskeletal Simulation and Exoskeleton Control.Biosensors (Basel). 2022 May 9;12(5):312. doi: 10.3390/bios12050312. Biosensors (Basel). 2022. PMID: 35624613 Free PMC article.
References
-
- An KN, Takahashi K, Harrigan TP, Chao EY. Determination of muscle orientations and moment arms. Journal of Biomechical Engineering. 1984;106:280–282. - PubMed
-
- Anderson FC, Pandy MG. Dynamic optimization of human walking. Journal of Biomechical Engineering. 2001;123:381–390. - PubMed
-
- Bouisset, S. (1973). EMG and muscle force in normal motor activities Basel: Karger.
-
- Buchanan TS. Evidence that maximum muscle stress is not a constant: Differences in specific tension in elbow flexors and extensors. Medical Engineering and Physics. 1995;17:529–536. - PubMed
-
- Buchanan TS, Almdale DPJ, Lewis JL, Rymer, WZ Characteristics of synergic relations during isometric contractions of human elbow muscles. Journal of Neurophysiology. 1986;5:1225–1241. - PubMed
Grants and funding
- P20 RR016458-02/RR/NCRR NIH HHS/United States
- R01 AR046386/AR/NIAMS NIH HHS/United States
- P20 RR016458-04/RR/NCRR NIH HHS/United States
- R01 AR046386-03/AR/NIAMS NIH HHS/United States
- R01 HD038582-02/HD/NICHD NIH HHS/United States
- P20 RR016458/RR/NCRR NIH HHS/United States
- R01 HD038582-01A2/HD/NICHD NIH HHS/United States
- P20 RR016458-03/RR/NCRR NIH HHS/United States
- R01 HD038582/HD/NICHD NIH HHS/United States
- R01 AR046386-04/AR/NIAMS NIH HHS/United States
- R01 HD038582-03/HD/NICHD NIH HHS/United States
- R01 AR046386-01/AR/NIAMS NIH HHS/United States
- P20 RR016458-01/RR/NCRR NIH HHS/United States
- R01 AR046386-05/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

