Gangliosides play an important role in the organization of CD82-enriched microdomains
- PMID: 16859490
- PMCID: PMC1652826
- DOI: 10.1042/BJ20060259
Gangliosides play an important role in the organization of CD82-enriched microdomains
Abstract
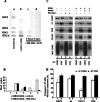
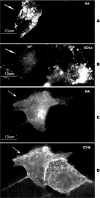
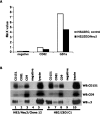
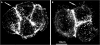
Four-transmembrane-domain proteins of the tetraspanin superfamily are the organizers of specific microdomains at the membrane [TERMs (tetraspanin-enriched microdomains)] that incorporate various transmembrane receptors and modulate their activities. The structural aspects of the organization of TERM are poorly understood. In the present study, we investigated the role of gangliosides in the assembly and stability of TERM. We demonstrated that inhibition of the glycosphingolipid biosynthetic pathway with specific inhibitors of glucosylceramide synthase [NB-DGJ (N-butyldeoxygalactonojirimycin) and PPMP (D-threo-1-phenyl-2-hexadecanoylamino-3-morpholino-1-propanol.HCl)] resulted in specific weakening of the interactions involving tetraspanin CD82. Furthermore, ectopic expression of the plasma-membrane-bound sialidase Neu3 in mammary epithelial cells also affected stability of the complexes containing CD82: its association with tetraspanin CD151 was decreased, but the association with EGFR [EGF (epidermal growth factor) receptor] was enhanced. The destabilization of the CD82-containing complexes upon ganglioside depletion correlated with the re-distribution of the proteins within plasma membrane. Importantly, depletion of gangliosides affected EGF-induced signalling only in the presence of CD82. Taken together, our results provide strong evidence that gangliosides play an important role in supporting the integrity of CD82-enriched microdomains. Furthermore, these results demonstrate that the association between different tetraspanins in TERM is controlled by distinct mechanisms and identify Neu3 as a first physiological regulator of the integrity of these microdomains.
Figures





Similar articles
-
CD82 and Gangliosides Tune CD81 Membrane Behavior.Int J Mol Sci. 2021 Aug 6;22(16):8459. doi: 10.3390/ijms22168459. Int J Mol Sci. 2021. PMID: 34445169 Free PMC article.
-
Synergistic inhibition of cell migration by tetraspanin CD82 and gangliosides occurs via the EGFR or cMet-activated Pl3K/Akt signalling pathway.Int J Biochem Cell Biol. 2013 Nov;45(11):2349-58. doi: 10.1016/j.biocel.2013.08.002. Epub 2013 Aug 19. Int J Biochem Cell Biol. 2013. PMID: 23968914
-
Control of cell motility by interaction of gangliosides, tetraspanins, and epidermal growth factor receptor in A431 versus KB epidermoid tumor cells.Carbohydr Res. 2009 Aug 17;344(12):1479-86. doi: 10.1016/j.carres.2009.04.032. Epub 2009 May 13. Carbohydr Res. 2009. PMID: 19559406
-
Gangliosides as regulators of cell membrane organization and functions.Adv Exp Med Biol. 2010;688:165-84. doi: 10.1007/978-1-4419-6741-1_12. Adv Exp Med Biol. 2010. PMID: 20919654 Review.
-
Functional role of glycosphingolipids and gangliosides in control of cell adhesion, motility, and growth, through glycosynaptic microdomains.Biochim Biophys Acta. 2008 Mar;1780(3):421-33. doi: 10.1016/j.bbagen.2007.10.008. Epub 2007 Oct 22. Biochim Biophys Acta. 2008. PMID: 17991443 Free PMC article. Review.
Cited by
-
Tetraspanins: push and pull in suppressing and promoting metastasis.Nat Rev Cancer. 2009 Jan;9(1):40-55. doi: 10.1038/nrc2543. Epub 2008 Dec 11. Nat Rev Cancer. 2009. PMID: 19078974 Review.
-
The Role of Glycosphingolipids in Immune Cell Functions.Front Immunol. 2019 Jan 29;10:90. doi: 10.3389/fimmu.2019.00090. eCollection 2019. Front Immunol. 2019. PMID: 30761148 Free PMC article. Review.
-
Function of Platelet Glycosphingolipid Microdomains/Lipid Rafts.Int J Mol Sci. 2020 Aug 2;21(15):5539. doi: 10.3390/ijms21155539. Int J Mol Sci. 2020. PMID: 32748854 Free PMC article. Review.
-
Actin-regulated Siglec-1 nanoclustering influences HIV-1 capture and virus-containing compartment formation in dendritic cells.Elife. 2023 Mar 20;12:e78836. doi: 10.7554/eLife.78836. Elife. 2023. PMID: 36940134 Free PMC article.
-
Mechanical Control of Cell Migration by the Metastasis Suppressor Tetraspanin CD82/KAI1.Cells. 2021 Jun 18;10(6):1545. doi: 10.3390/cells10061545. Cells. 2021. PMID: 34207462 Free PMC article.
References
-
- Brown D. A., Jacobson K. Microdomains, lipid rafts and caveolae (San Feliu de Guixols, Spain, 19–24 May 2001) Traffic. 2001;2:668–672. - PubMed
-
- Rajendran L., Simons K. Lipid rafts and membrane dynamics. J. Cell Sci. 2005;118:1099–1102. - PubMed
-
- Razani B., Woodman S. E., Lisanti M. P. Caveolae: from cell biology to animal physiology. Pharmacol. Rev. 2002;54:431–467. - PubMed
-
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

