Altered adenosine-to-inosine RNA editing in human cancer
- PMID: 17908822
- PMCID: PMC2045141
- DOI: 10.1101/gr.6493107
Altered adenosine-to-inosine RNA editing in human cancer
Abstract
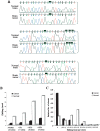
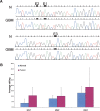
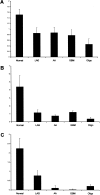
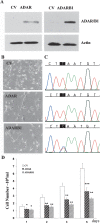
Adenosine-to-inosine (A-to-I) RNA editing was recently shown to be abundant in the human transcriptome, affecting thousands of genes. Employing a bioinformatic approach, we identified significant global hypoediting of Alu repetitive elements in brain, prostate, lung, kidney, and testis tumors. Experimental validation confirmed this finding, showing significantly reduced editing in Alu sequences within MED13 transcripts in brain tissues. Looking at editing of specific recoding and noncoding sites, including in cancer-related genes, a more complex picture emerged, with a gene-specific editing pattern in tumors vs. normal tissues. Additionally, we found reduced RNA levels of all three editing mediating enzymes, ADAR, ADARB1, and ADARB2, in brain tumors. The reduction of ADARB2 correlated with the grade of malignancy of glioblastoma multiforme, the most aggressive of brain tumors, displaying a 99% decrease in ADARB2 RNA levels. Consistently, overexpression of ADAR and ADARB1 in the U87 glioblastoma multiforme cell line resulted in decreased proliferation rate, suggesting that reduced A-to-I editing in brain tumors is involved in the pathogenesis of cancer. Altered epigenetic control was recently shown to play a central role in oncogenesis. We suggest that A-to-I RNA editing may serve as an additional epigenetic mechanism relevant to cancer development and progression.
Figures

Similar articles
-
Comparative genomic and bioinformatic approaches for the identification of new adenosine-to-inosine substrates.Methods Enzymol. 2007;424:245-64. doi: 10.1016/S0076-6879(07)24011-X. Methods Enzymol. 2007. PMID: 17662844
-
Widespread A-to-I RNA editing of Alu-containing mRNAs in the human transcriptome.PLoS Biol. 2004 Dec;2(12):e391. doi: 10.1371/journal.pbio.0020391. Epub 2004 Nov 9. PLoS Biol. 2004. PMID: 15534692 Free PMC article.
-
Alu sequences in undifferentiated human embryonic stem cells display high levels of A-to-I RNA editing.PLoS One. 2010 Jun 21;5(6):e11173. doi: 10.1371/journal.pone.0011173. PLoS One. 2010. PMID: 20574523 Free PMC article.
-
In cancer, A-to-I RNA editing can be the driver, the passenger, or the mechanic.Drug Resist Updat. 2017 May;32:16-22. doi: 10.1016/j.drup.2017.09.001. Epub 2017 Oct 4. Drug Resist Updat. 2017. PMID: 29145975 Review.
-
Adenosine-to-inosine RNA editing meets cancer.Carcinogenesis. 2011 Nov;32(11):1569-77. doi: 10.1093/carcin/bgr124. Epub 2011 Jun 29. Carcinogenesis. 2011. PMID: 21715563 Review.
Cited by
-
Identification of prognostic RNA editing profiles for clear cell renal carcinoma.Front Med (Lausanne). 2024 Jul 18;11:1390803. doi: 10.3389/fmed.2024.1390803. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39091293 Free PMC article.
-
Spatial Visualization of A-to-I Editing in Cells Using Endonuclease V Immunostaining Assay (EndoVIA).ACS Cent Sci. 2024 Jul 8;10(7):1396-1405. doi: 10.1021/acscentsci.4c00444. eCollection 2024 Jul 24. ACS Cent Sci. 2024. PMID: 39071059 Free PMC article.
-
A-to-I Editing Is Subtype-Specific in Non-Hodgkin Lymphomas.Genes (Basel). 2024 Jul 1;15(7):864. doi: 10.3390/genes15070864. Genes (Basel). 2024. PMID: 39062643 Free PMC article.
-
ADBP-1 regulates ADR-2 nuclear localization to control editing substrate selection.Nucleic Acids Res. 2024 Sep 9;52(16):9501-9518. doi: 10.1093/nar/gkae641. Nucleic Acids Res. 2024. PMID: 39036970 Free PMC article.
-
Development and assessment of an RNA editing-based risk model for the prognosis of cervical cancer patients.Medicine (Baltimore). 2024 May 10;103(19):e38116. doi: 10.1097/MD.0000000000038116. Medicine (Baltimore). 2024. PMID: 38728474 Free PMC article.
References
-
- Bhalla K.N. Epigenetic and chromatin modifiers as _targeted therapy of hematologic malignancies. J. Clin. Oncol. 2005;23:3971–3993. - PubMed
-
- Blow M.J., Grocock R.J., van Dongen S., Enright A.J., Dicks E., Futreal P.A., Wooster R., Stratton M.R., Grocock R.J., van Dongen S., Enright A.J., Dicks E., Futreal P.A., Wooster R., Stratton M.R., van Dongen S., Enright A.J., Dicks E., Futreal P.A., Wooster R., Stratton M.R., Enright A.J., Dicks E., Futreal P.A., Wooster R., Stratton M.R., Dicks E., Futreal P.A., Wooster R., Stratton M.R., Futreal P.A., Wooster R., Stratton M.R., Wooster R., Stratton M.R., Stratton M.R. RNA editing of human microRNAs. Genome Biol. 2006;7:R27. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
