Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway
- PMID: 18434384
- PMCID: PMC2494502
- DOI: 10.1152/ajprenal.00577.2007
Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-beta1 pathway
Abstract
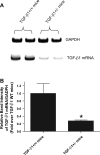
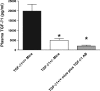
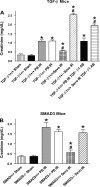
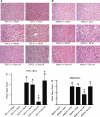
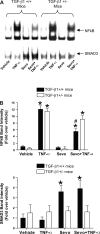
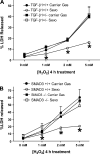
We previously demonstrated that several clinically utilized volatile anesthetics including sevoflurane protected against renal ischemia-reperfusion (IR) injury by reducing necrosis and inflammation in vivo. We also demonstrated that volatile anesthetics produced direct anti-necrotic and anti-inflammatory effects in cultured renal tubules via mechanisms involving the externalization of phosphatidylserine and subsequent release of transforming growth factor (TGF)-beta1. In this study, we tested the hypothesis that volatile anesthetic-mediated renal protection requires TGF-beta1 and SMAD3 signaling in vivo. We subjected TGF-beta1+/+, TGF-beta1+/-, SMAD3+/+, or SMAD3-/- mice to renal IR under anesthesia with pentobarbital sodium or with sevoflurane. Although TGF-beta1+/+ and SMAD3+/+ mice were significantly protected against renal IR injury under sevoflurane anesthesia with reduced necrosis and inflammation, TGF-beta1+/- mice and SMAD3-/- mice were not protected against renal IR with sevoflurane. Furthermore, a neutralizing TGF-beta1 antibody blocked renal protection with sevoflurane in TGF-beta1+/+ mice. Sevoflurane caused nuclear translocation of SMAD3 and reduced the TNF-alpha-induced nuclear translocation of NF-kappaB in primary cultures of proximal tubules from TGF-beta1+/+ but not in TGF-beta1+/- mice. Finally, sevoflurane protected against necrosis induced with hydrogen peroxide in primary cultures of proximal tubules from TGF-beta1+/+ mice or SMAD3+/+ mice but not in proximal tubules from TGF-beta1+/- or SMAD3-/- mice. Therefore, we demonstrate in this study that sevoflurane-mediated renal protection in vivo requires the TGF-beta1-->SMAD3 signaling pathway.
Figures
Similar articles
-
The volatile anesthetic isoflurane induces ecto-5'-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury.Kidney Int. 2013 Jul;84(1):90-103. doi: 10.1038/ki.2013.43. Epub 2013 Feb 20. Kidney Int. 2013. PMID: 23423261 Free PMC article.
-
Volatile anesthetics and AKI: risks, mechanisms, and a potential therapeutic window.J Am Soc Nephrol. 2014 May;25(5):884-92. doi: 10.1681/ASN.2013111215. Epub 2014 Feb 7. J Am Soc Nephrol. 2014. PMID: 24511126 Free PMC article. Review.
-
Sevoflurane-mediated TGF-beta1 signaling in renal proximal tubule cells.Am J Physiol Renal Physiol. 2008 Feb;294(2):F371-8. doi: 10.1152/ajprenal.00277.2007. Epub 2007 Dec 5. Am J Physiol Renal Physiol. 2008. PMID: 18057187
-
Anti-inflammatory and antinecrotic effects of the volatile anesthetic sevoflurane in kidney proximal tubule cells.Am J Physiol Renal Physiol. 2006 Jul;291(1):F67-78. doi: 10.1152/ajprenal.00412.2005. Epub 2006 Feb 14. Am J Physiol Renal Physiol. 2006. PMID: 16478975
-
Central role of dysregulation of TGF-β/Smad in CKD progression and potential _targets of its treatment.Biomed Pharmacother. 2018 May;101:670-681. doi: 10.1016/j.biopha.2018.02.090. Epub 2018 Mar 22. Biomed Pharmacother. 2018. PMID: 29518614 Review.
Cited by
-
The volatile anesthetic isoflurane induces ecto-5'-nucleotidase (CD73) to protect against renal ischemia and reperfusion injury.Kidney Int. 2013 Jul;84(1):90-103. doi: 10.1038/ki.2013.43. Epub 2013 Feb 20. Kidney Int. 2013. PMID: 23423261 Free PMC article.
-
Impact of anesthesia, analgesia, and euthanasia technique on the inflammatory cytokine profile in a rodent model of severe burn injury.Shock. 2010 Sep;34(3):261-8. doi: 10.1097/shk.0b013e3181d8e2a6. Shock. 2010. PMID: 20803788 Free PMC article.
-
Critical role of interleukin-11 in isoflurane-mediated protection against ischemic acute kidney injury in mice.Anesthesiology. 2013 Dec;119(6):1389-401. doi: 10.1097/ALN.0b013e3182a950da. Anesthesiology. 2013. PMID: 24037316 Free PMC article.
-
Volatile anesthetics and AKI: risks, mechanisms, and a potential therapeutic window.J Am Soc Nephrol. 2014 May;25(5):884-92. doi: 10.1681/ASN.2013111215. Epub 2014 Feb 7. J Am Soc Nephrol. 2014. PMID: 24511126 Free PMC article. Review.
-
Specific microRNAs are involved in the reno‑protective effects of sevoflurane preconditioning and ischemic preconditioning against ischemia reperfusion injury in rats.Int J Mol Med. 2020 Apr;45(4):1141-1149. doi: 10.3892/ijmm.2020.4477. Epub 2020 Jan 27. Int J Mol Med. 2020. PMID: 31985019 Free PMC article.
References
-
- Bove T, Calabro MG, Landoni G, Aletti G, Marino G, Crescenzi G, Rosica C, Zangrillo A. The incidence and risk of acute renal failure after cardiac surgery. J Cardiothorac Vasc Anesth 18: 442–445, 2004. - PubMed
-
- Chertow GM, Lazarus JM, Christiansen CL, Cook EF, Hammermeister KE, Grover F, Daley J. Preoperative renal risk stratification. Circulation 95: 878–884, 1997. - PubMed
-
- Conlon PJ, Stafford-Smith M, White WD, Newman MF, King S, Winn MP, Landolfo K. Acute renal failure following cardiac surgery. Nephrol Dial Transplant 14: 1158–1162, 1999. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

