Reactive oxygen species, cellular redox systems, and apoptosis
- PMID: 20045723
- PMCID: PMC2823977
- DOI: 10.1016/j.freeradbiomed.2009.12.022
Reactive oxygen species, cellular redox systems, and apoptosis
Abstract
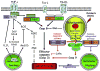
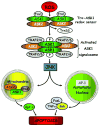
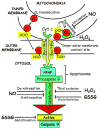
Reactive oxygen species (ROS) are products of normal metabolism and xenobiotic exposure, and depending on their concentration, ROS can be beneficial or harmful to cells and tissues. At physiological low levels, ROS function as "redox messengers" in intracellular signaling and regulation, whereas excess ROS induce oxidative modification of cellular macromolecules, inhibit protein function, and promote cell death. Additionally, various redox systems, such as the glutathione, thioredoxin, and pyridine nucleotide redox couples, participate in cell signaling and modulation of cell function, including apoptotic cell death. Cell apoptosis is initiated by extracellular and intracellular signals via two main pathways, the death receptor- and the mitochondria-mediated pathways. Various pathologies can result from oxidative stress-induced apoptotic signaling that is consequent to ROS increases and/or antioxidant decreases, disruption of intracellular redox homeostasis, and irreversible oxidative modifications of lipid, protein, or DNA. In this review, we focus on several key aspects of ROS and redox mechanisms in apoptotic signaling and highlight the gaps in knowledge and potential avenues for further investigation. A full understanding of the redox control of apoptotic initiation and execution could underpin the development of therapeutic interventions _targeted at oxidative stress-associated disorders.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
[Selenium compounds in redox regulation of inflammation and apoptosis].Biomed Khim. 2019 Apr;65(3):165-179. doi: 10.18097/PBMC20196503165. Biomed Khim. 2019. PMID: 31258141 Review. Russian.
-
Spatio-temporal changes in glutathione and thioredoxin redox couples during ionizing radiation-induced oxidative stress regulate tumor radio-resistance.Free Radic Res. 2015 Oct;49(10):1218-32. doi: 10.3109/10715762.2015.1056180. Free Radic Res. 2015. PMID: 26021764
-
Cellular thiols and reactive oxygen species in drug-induced apoptosis.J Pharmacol Exp Ther. 2001 Jan;296(1):1-6. J Pharmacol Exp Ther. 2001. PMID: 11123355 Review.
-
Redox control of cell death.Antioxid Redox Signal. 2002 Jun;4(3):405-14. doi: 10.1089/15230860260196209. Antioxid Redox Signal. 2002. PMID: 12215208 Review.
-
Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling.Cell Signal. 2012 May;24(5):981-90. doi: 10.1016/j.cellsig.2012.01.008. Epub 2012 Jan 20. Cell Signal. 2012. PMID: 22286106 Free PMC article. Review.
Cited by
-
Does Tramadol Exposure Have Unfavorable Effects on Hippocampus? A Review Study.Addict Health. 2024 Jul;16(3):213-223. doi: 10.34172/ahj.1481. Epub 2024 Jul 29. Addict Health. 2024. PMID: 39439859 Free PMC article. Review.
-
Non-Esterified Fatty Acid-Induced Reactive Oxygen Species Mediated Granulosa Cells Apoptosis Is Regulated by Nrf2/p53 Signaling Pathway.Antioxidants (Basel). 2020 Jun 14;9(6):523. doi: 10.3390/antiox9060523. Antioxidants (Basel). 2020. PMID: 32545880 Free PMC article.
-
Formaldehyde induces toxicity in mouse bone marrow and hematopoietic stem/progenitor cells and enhances benzene-induced adverse effects.Arch Toxicol. 2017 Feb;91(2):921-933. doi: 10.1007/s00204-016-1760-5. Epub 2016 Jun 23. Arch Toxicol. 2017. PMID: 27339418 Free PMC article.
-
Avian Coronavirus Infectious Bronchitis Virus Activates Mitochondria-Mediated Apoptosis Pathway and Affects Viral Replication by Inducing Reactive Oxygen Species Production in Chicken HD11 Cells.Biology (Basel). 2024 Jul 1;13(7):491. doi: 10.3390/biology13070491. Biology (Basel). 2024. PMID: 39056685 Free PMC article.
-
Thiol/Disulfide Homeostasis in Bipolar and Unipolar Depression.Clin Psychopharmacol Neurosci. 2020 Aug 31;18(3):395-401. doi: 10.9758/cpn.2020.18.3.395. Clin Psychopharmacol Neurosci. 2020. PMID: 32702218 Free PMC article.
References
-
- Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–214. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

