AMPK as a metabolic tumor suppressor: control of metabolism and cell growth
- PMID: 20222801
- PMCID: PMC2854547
- DOI: 10.2217/fon.09.174
AMPK as a metabolic tumor suppressor: control of metabolism and cell growth
Abstract
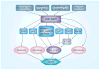
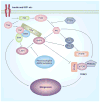
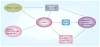
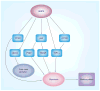
AMPK is an evolutionarily conserved fuel-sensing enzyme that is activated in shortage of energy and suppressed in its surfeit. AMPK activation stimulates fatty acid oxidation, enhances insulin sensitivity, alleviates hyperglycemia and hyperlipidemia, and inhibits proinflammatory changes. Thus, AMPK is a well-received therapeutic _target for metabolic syndrome and Type 2 diabetes. Recent studies indicate that AMPK plays a role in linking metabolic syndrome and cancer. AMPK is an essential mediator of the tumor suppressor LKB1 and could be suppressed in cancer cells containing loss-of-function mutations of LKB1 or containing active mutations of B-Raf, or in cancers associated with metabolic syndrome. The activation of AMPK reprograms cellular metabolism and enforces metabolic checkpoints by acting on mTORC1, p53, fatty acid synthase and other molecules for regulating cell growth and metabolism. In keeping with in vitro studies, recent epidemiological studies indicate that the incidence of cancer is reduced in Type 2 diabetes treated with metformin, an AMPK activator. Thus, AMPK is emerging as an interesting metabolic tumor suppressor and a promising _target for cancer prevention and therapy.
Figures
Similar articles
-
_targeting AMPK for cancer prevention and treatment.Onco_target. 2015 Apr 10;6(10):7365-78. doi: 10.18632/onco_target.3629. Onco_target. 2015. PMID: 25812084 Free PMC article. Review.
-
LKB1; linking cell structure and tumor suppression.Oncogene. 2008 Nov 24;27(55):6908-19. doi: 10.1038/onc.2008.342. Oncogene. 2008. PMID: 19029933 Review.
-
The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator.Cancer Lett. 2015 Jan 28;356(2 Pt A):165-70. doi: 10.1016/j.canlet.2014.01.018. Epub 2014 Jan 28. Cancer Lett. 2015. PMID: 24486219 Review.
-
AMPK, the metabolic syndrome and cancer.Trends Pharmacol Sci. 2005 Feb;26(2):69-76. doi: 10.1016/j.tips.2004.12.011. Trends Pharmacol Sci. 2005. PMID: 15681023 Review.
-
A new role of NUAK1: directly phosphorylating p53 and regulating cell proliferation.Oncogene. 2011 Jun 30;30(26):2933-42. doi: 10.1038/onc.2011.19. Epub 2011 Feb 14. Oncogene. 2011. PMID: 21317932
Cited by
-
Minireview: Obesity and breast cancer: a tale of inflammation and dysregulated metabolism.Mol Endocrinol. 2013 May;27(5):715-25. doi: 10.1210/me.2013-1011. Epub 2013 Mar 21. Mol Endocrinol. 2013. PMID: 23518927 Free PMC article. Review.
-
Emerging glycolysis _targeting and drug discovery from chinese medicine in cancer therapy.Evid Based Complement Alternat Med. 2012;2012:873175. doi: 10.1155/2012/873175. Epub 2012 Jul 12. Evid Based Complement Alternat Med. 2012. PMID: 22844340 Free PMC article.
-
Synergic chemoprevention with dietary carbohydrate restriction and supplementation of AMPK-activating phytochemicals: the role of SIRT1.Eur J Cancer Prev. 2016 Jan;25(1):54-64. doi: 10.1097/CEJ.0000000000000141. Eur J Cancer Prev. 2016. PMID: 25747515 Free PMC article.
-
Resveratrol induces AMPK-dependent MDR1 inhibition in colorectal cancer HCT116/L-OHP cells by preventing activation of NF-κB signaling and suppressing cAMP-responsive element transcriptional activity.Tumour Biol. 2015 Dec;36(12):9499-510. doi: 10.1007/s13277-015-3636-3. Epub 2015 Jul 1. Tumour Biol. 2015. PMID: 26124005
-
Yeast and cancer cells - common principles in lipid metabolism.Biochim Biophys Acta. 2013 Feb;1831(2):314-26. doi: 10.1016/j.bbalip.2012.09.003. Epub 2012 Sep 16. Biochim Biophys Acta. 2013. PMID: 22989772 Free PMC article. Review.
References
-
- Steinberg GR, Kemp BE. AMPK in health and Disease. Physiol Rev. 2009;89(3):1025–1078. - PubMed
-
- Ruderman N, Prentki M. AMP kinase and malonyl-CoA: _targets for therapy of the metabolic syndrome. Nat Rev Drug Discov. 2004;3(4):340–351. - PubMed
-
- Luo Z, Saha AK, Xiang X, Ruderman NB. AMPK, the metabolic syndrome and cancer. Trends Pharmacol Sci. 2005;26(2):69–76. - PubMed
-
- Hoyer-Hansen M, Jaattela M. AMP-activated protein kinase: a universal regulator of autophagy? Autophagy. 2007;3(4):381–383. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
