STAT3 controls myeloid progenitor growth during emergency granulopoiesis
- PMID: 20581311
- PMCID: PMC2953883
- DOI: 10.1182/blood-2009-12-259630
STAT3 controls myeloid progenitor growth during emergency granulopoiesis
Abstract
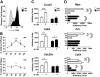
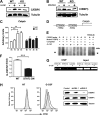
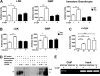
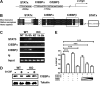
Granulocyte colony-stimulating factor (G-CSF) mediates "emergency" granulopoiesis during infection, a process that is mimicked by clinical G-CSF use, yet we understand little about the intracellular signaling cascades that control demand-driven neutrophil production. Using a murine model with conditional deletion of signal transducer and activator of transcription 3 (STAT3) in bone marrow, we investigated the cellular and molecular mechanisms of STAT3 function in the emergency granulopoiesis response to G-CSF administration or infection with Listeria monocytogenes, a pathogen that is restrained by G-CSF signaling in vivo. Our results show that STAT3 deficiency renders hematopoietic progenitor cells and myeloid precursors refractory to the growth-promoting functions of G-CSF or L monocytogenes infection. STAT3 is necessary for accelerating granulocyte cell-cycle progression and maturation in response to G-CSF. STAT3 directly controls G-CSF-dependent expression of CCAAT-enhancer-binding protein β (C/EBPβ), a crucial factor in the emergency granulopoiesis response. Moreover, STAT3 and C/EBPβ coregulate c-Myc through interactions with the c-myc promoter that control the duration of C/EBPα occupancy during demand-driven granulopoiesis. These results place STAT3 as an essential mediator of emergency granulopoiesis by its regulation of transcription factors that direct G-CSF-responsive myeloid progenitor expansion.
Figures


Similar articles
-
Signal transducers and activators of transcription 3 augments the transcriptional activity of CCAAT/enhancer-binding protein alpha in granulocyte colony-stimulating factor signaling pathway.J Biol Chem. 2005 Apr 1;280(13):12621-9. doi: 10.1074/jbc.M408442200. Epub 2005 Jan 21. J Biol Chem. 2005. PMID: 15664994
-
CCAAT/enhancer-binding proteins are required for granulopoiesis independent of their induction of the granulocyte colony-stimulating factor receptor.Blood. 2002 Apr 15;99(8):2776-85. doi: 10.1182/blood.v99.8.2776. Blood. 2002. PMID: 11929766
-
Stat3 and CCAAT enhancer-binding protein β (C/ebpβ) activate Fanconi C gene transcription during emergency granulopoiesis.J Biol Chem. 2018 Mar 16;293(11):3937-3948. doi: 10.1074/jbc.RA117.000528. Epub 2018 Jan 30. J Biol Chem. 2018. PMID: 29382715 Free PMC article.
-
The granulocyte colony-stimulating factor receptor and its role in disorders of granulopoiesis.Leuk Lymphoma. 1998 Jan;28(3-4):265-73. doi: 10.3109/10428199809092682. Leuk Lymphoma. 1998. PMID: 9517498 Review.
-
Stat3 and G-CSF-induced myeloid differentiation.Leuk Lymphoma. 1998 Aug;30(5-6):433-42. doi: 10.3109/10428199809057555. Leuk Lymphoma. 1998. PMID: 9711905 Review.
Cited by
-
Relevance of Interferon Regulatory Factor-8 Expression in Myeloid-Tumor Interactions.J Interferon Cytokine Res. 2016 Jul;36(7):442-53. doi: 10.1089/jir.2015.0174. J Interferon Cytokine Res. 2016. PMID: 27379866 Free PMC article. Review.
-
Human STAT3 variants underlie autosomal dominant hyper-IgE syndrome by negative dominance.J Exp Med. 2021 Aug 2;218(8):e20202592. doi: 10.1084/jem.20202592. Epub 2021 Jun 17. J Exp Med. 2021. PMID: 34137790 Free PMC article.
-
Coordinated regulation of myeloid cells by tumours.Nat Rev Immunol. 2012 Mar 22;12(4):253-68. doi: 10.1038/nri3175. Nat Rev Immunol. 2012. PMID: 22437938 Free PMC article. Review.
-
Genetic rescue of lineage-balanced blood cell production reveals a crucial role for STAT3 antiinflammatory activity in hematopoiesis.Proc Natl Acad Sci U S A. 2018 Mar 6;115(10):E2311-E2319. doi: 10.1073/pnas.1713889115. Epub 2018 Feb 20. Proc Natl Acad Sci U S A. 2018. PMID: 29463696 Free PMC article.
-
Noncanonical NF-κB activation mediates STAT3-stimulated IDO upregulation in myeloid-derived suppressor cells in breast cancer.J Immunol. 2014 Sep 1;193(5):2574-86. doi: 10.4049/jimmunol.1400833. Epub 2014 Jul 25. J Immunol. 2014. PMID: 25063873 Free PMC article. Clinical Trial.
References
-
- Boxer L, Dale DC. Neutropenia: causes and consequences. Semin Hematol. 2002;39(2):75–81. - PubMed
-
- Eyles JL, Hickey MJ, Norman MU, et al. A key role for G-CSF-induced neutrophil production and trafficking during inflammatory arthritis. Blood. 2008;112(13):5193–5201. - PubMed
-
- Al-Gwaiz LA, Babay HH. The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections. Med Princ Pract. 2007;16(5):344–347. - PubMed
-
- McKinstry WJ, Li CL, Rasko JE, Nicola NA, Johnson GR, Metcalf D. Cytokine receptor expression on hematopoietic stem and progenitor cells. Blood. 1997;89(1):65–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

