Candida albicans forms biofilms on the vaginal mucosa
- PMID: 20705667
- PMCID: PMC3068702
- DOI: 10.1099/mic.0.039354-0
Candida albicans forms biofilms on the vaginal mucosa
Abstract
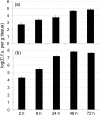
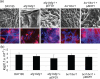
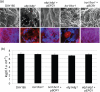
Current understanding of resistance and susceptibility to vulvovaginal candidiasis challenges existing paradigms of host defence against fungal infection. While abiotic biofilm formation has a clearly established role during systemic Candida infections, it is not known whether C. albicans forms biofilms on the vaginal mucosa and the possible role of biofilms in disease. In vivo and ex vivo murine vaginitis models were employed to examine biofilm formation by scanning electron and confocal microscopy. C. albicans strains included 3153A (lab strain), DAY185 (parental control strain), and mutants defective in morphogenesis and/or biofilm formation in vitro (efg1/efg1 and bcr1/bcr1). Both 3153A and DAY815 formed biofilms on the vaginal mucosa in vivo and ex vivo as indicated by high fungal burden and microscopic analysis demonstrating typical biofilm architecture and presence of extracellular matrix (ECM) co-localized with the presence of fungi. In contrast, efg1/efg1 and bcr1/bcr1 mutant strains exhibited weak or no biofilm formation/ECM production in both models compared to wild-type strains and complemented mutants despite comparable colonization levels. These data show for the first time that C. albicans forms biofilms in vivo on vaginal epithelium, and that in vivo biotic biofilm formation requires regulators of biofilm formation (BCR1) and morphogenesis (EFG1).
Figures


Similar articles
-
Transcription Factors Efg1 and Bcr1 Regulate Biofilm Formation and Virulence during Candida albicans-Associated Denture Stomatitis.PLoS One. 2016 Jul 25;11(7):e0159692. doi: 10.1371/journal.pone.0159692. eCollection 2016. PLoS One. 2016. PMID: 27453977 Free PMC article.
-
Role of SFP1 in the Regulation of Candida albicans Biofilm Formation.PLoS One. 2015 Jun 18;10(6):e0129903. doi: 10.1371/journal.pone.0129903. eCollection 2015. PLoS One. 2015. PMID: 26087243 Free PMC article.
-
S. oralis activates the Efg1 filamentation pathway in C. albicans to promote cross-kingdom interactions and mucosal biofilms.Virulence. 2017 Nov 17;8(8):1602-1617. doi: 10.1080/21505594.2017.1326438. Epub 2017 Jun 1. Virulence. 2017. PMID: 28481721 Free PMC article.
-
Candida albicans biofilms and polymicrobial interactions.Crit Rev Microbiol. 2021 Feb;47(1):91-111. doi: 10.1080/1040841X.2020.1843400. Epub 2021 Jan 22. Crit Rev Microbiol. 2021. PMID: 33482069 Free PMC article. Review.
-
Pathogenesis of Candida albicans biofilm.Pathog Dis. 2016 Jun;74(4):ftw018. doi: 10.1093/femspd/ftw018. Pathog Dis. 2016. PMID: 26960943 Free PMC article. Review.
Cited by
-
The SPS amino acid sensor mediates nutrient acquisition and immune evasion in Candida albicans.Cell Microbiol. 2016 Nov;18(11):1611-1624. doi: 10.1111/cmi.12600. Epub 2016 May 27. Cell Microbiol. 2016. PMID: 27060451 Free PMC article.
-
Waikialoid A suppresses hyphal morphogenesis and inhibits biofilm development in pathogenic Candida albicans.J Nat Prod. 2012 Apr 27;75(4):707-15. doi: 10.1021/np2009994. Epub 2012 Mar 8. J Nat Prod. 2012. PMID: 22400916 Free PMC article.
-
O-mannosylation in Candida albicans enables development of interkingdom biofilm communities.mBio. 2014 Apr 15;5(2):e00911. doi: 10.1128/mBio.00911-14. mBio. 2014. PMID: 24736223 Free PMC article.
-
Candida Species Biofilms' Antifungal Resistance.J Fungi (Basel). 2017 Feb 21;3(1):8. doi: 10.3390/jof3010008. J Fungi (Basel). 2017. PMID: 29371527 Free PMC article. Review.
-
The Host's Reply to Candida Biofilm.Pathogens. 2016 Mar 18;5(1):33. doi: 10.3390/pathogens5010033. Pathogens. 2016. PMID: 26999221 Free PMC article. Review.
References
-
- Baillie, G. S. & Douglas, L. J. (2000). Matrix polymers of Candida biofilms and their possible role in biofilm resistance to antifungal agents. J Antimicrob Chemother 46, 397–403. - PubMed
-
- Barousse, M. M., Steele, C., Dunlap, K., Espinosa, T., Boikov, D., Sobel, J. D. & Fidel, P. L., Jr (2001). Growth inhibition of Candida albicans by human vaginal epithelial cells. J Infect Dis 184, 1489–1493. - PubMed
-
- Bauters, T. G., Dhont, M. A., Temmerman, M. I. & Nelis, H. J. (2002). Prevalence of vulvovaginal candidiasis and susceptibility to fluconazole in women. Am J Obstet Gynecol 187, 569–574. - PubMed
-
- Blankenship, J. R. & Mitchell, A. P. (2006). How to build a biofilm: a fungal perspective. Curr Opin Microbiol 9, 588–594. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

