Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation
- PMID: 20852386
- PMCID: PMC2947238
- DOI: 10.1172/JCI43343
Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation
Abstract
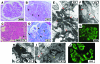
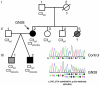
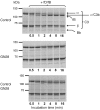
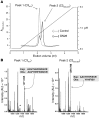
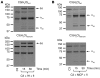
Dense deposit disease (DDD) is a severe renal disease characterized by accumulation of electron-dense material in the mesangium and glomerular basement membrane. Previously, DDD has been associated with deficiency of factor H (fH), a plasma regulator of the alternative pathway (AP) of complement activation, and studies in animal models have linked pathogenesis to the massive complement factor 3 (C3) activation caused by this deficiency. Here, we identified a unique DDD pedigree that associates disease with a mutation in the C3 gene. Mutant C(3923ΔDG), which lacks 2 amino acids, could not be cleaved to C3b by the AP C3-convertase and was therefore the predominant circulating C3 protein in the patients. However, upon activation to C3b by proteases, or to C3(H₂O) by spontaneous thioester hydrolysis, C(3923ΔDG) generated an active AP C3-convertase that was regulated normally by decay accelerating factor (DAF) but was resistant to decay by fH. Moreover, activated C(3b923ΔDG) and C3(H₂O)(923ΔDG) were resistant to proteolysis by factor I (fI) in the presence of fH, but were efficiently inactivated in the presence of membrane cofactor protein (MCP). These characteristics cause a fluid phase-restricted AP dysregulation in the patients that continuously activated and consumed C3 produced by the normal C3 allele. These findings expose structural requirements in C3 that are critical for recognition of the substrate C3 by the AP C3-convertase and for the regulatory activities of fH, DAF, and MCP, all of which have implications for therapeutic developments.
Figures
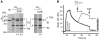
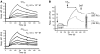



Similar articles
-
Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement.Clin J Am Soc Nephrol. 2011 May;6(5):1009-17. doi: 10.2215/CJN.07110810. Epub 2011 Mar 17. Clin J Am Soc Nephrol. 2011. PMID: 21415311 Free PMC article.
-
Generation of multiple fluid-phase C3b:plasma protein complexes during complement activation: possible implications in C3 glomerulopathies.J Immunol. 2014 Feb 1;192(3):1220-30. doi: 10.4049/jimmunol.1302288. Epub 2013 Dec 23. J Immunol. 2014. PMID: 24367026 Free PMC article.
-
Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies.Kidney Int. 2012 Aug;82(4):454-64. doi: 10.1038/ki.2012.63. Epub 2012 Mar 28. Kidney Int. 2012. PMID: 22456601
-
Hereditary and acquired complement dysregulation in membranoproliferative glomerulonephritis.Thromb Haemost. 2009 Feb;101(2):271-8. Thromb Haemost. 2009. PMID: 19190809 Review.
-
C3 glomerulopathy.Contrib Nephrol. 2013;181:185-93. doi: 10.1159/000348654. Epub 2013 May 8. Contrib Nephrol. 2013. PMID: 23689580 Review.
Cited by
-
C3 glomerulopathy-associated CFHR1 mutation alters FHR oligomerization and complement regulation.J Clin Invest. 2013 Jun;123(6):2434-46. doi: 10.1172/JCI68280. J Clin Invest. 2013. PMID: 23728178 Free PMC article.
-
A clinical approach to children with C3 glomerulopathy.Pediatr Nephrol. 2022 Mar;37(3):521-535. doi: 10.1007/s00467-021-05088-7. Epub 2021 May 18. Pediatr Nephrol. 2022. PMID: 34002292 Review.
-
Substrate recognition by complement convertases revealed in the C5-cobra venom factor complex.EMBO J. 2011 Feb 2;30(3):606-16. doi: 10.1038/emboj.2010.341. Epub 2011 Jan 7. EMBO J. 2011. PMID: 21217642 Free PMC article.
-
Dense deposit disease in an adolescent male mimicking acute post-streptococcal glomerulonephritis.Hippokratia. 2020 Oct-Dec;24(4):191-193. Hippokratia. 2020. PMID: 35023895 Free PMC article.
-
A Narrative Review on C3 Glomerulopathy: A Rare Renal Disease.Int J Mol Sci. 2020 Jan 14;21(2):525. doi: 10.3390/ijms21020525. Int J Mol Sci. 2020. PMID: 31947692 Free PMC article. Review.
References
-
- Lachmann PJ. The amplification loop of the complement pathways. Adv Immunol. 2009;104:115–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

