Effects of receptor binding on plasma half-life of bifunctional transferrin fusion proteins
- PMID: 21291258
- PMCID: PMC3310397
- DOI: 10.1021/mp1003064
Effects of receptor binding on plasma half-life of bifunctional transferrin fusion proteins
Abstract
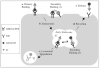
In contrast to the wide applications of recombinant bifunctional fusion proteins in clinical usage, the systematic study for the pharmacokinetics (PK) of bifunctional fusion proteins is left blank. In this report, recombinant fusion proteins consisting of transferrin (Tf) and growth hormone (GH) or granulocyte colony-stimulating factor (G-CSF) have been constructed as a model for studying the PK of bifunctional fusion proteins. The results showed that the insertion of different linkers between the two protein domains altered the binding affinities of the fusion proteins to both domain receptors, and that the fusion proteins' plasma half-lives were greatly affected. A strong correlation between GH receptor binding affinity and plasma half-life of GH-Tf fusion proteins was observed. In addition, we demonstrated that the intracellular processing after receptor binding plays an important role in determining the half-life of fusion proteins. While the binding of the GH domain to the GH receptor will lead to endocytosis and lysosomal degradation in _target cells, binding of the Tf domain to the Tf receptor may recycle the fusion protein and prolong its plasma half-life. To further confirm the effects of receptor binding on plasma half-life, G-CSF-Tf bifunctional fusion proteins with the same three linkers as GH-Tf were evaluated. While the 3 fusion proteins showed a similar G-CSF receptor binding affinity, the G-CSF-Tf fusion protein with the higher Tf receptor binding affinity exhibited longer plasma half-life. The linker insertion further demonstrated the involvement of Tf in recycling and prolonging plasma half-life. Based on our results, a model was developed to summarize the factors in determining the PK of bifunctional fusion proteins. Our findings are useful for predicting the plasma half-lives, as well as for improving the pharmacokinetic profiles of therapeutic bifunctional fusion proteins by applying linker technology.
Figures



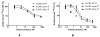


Similar articles
-
Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization.Pharm Res. 2006 Sep;23(9):2116-21. doi: 10.1007/s11095-006-9059-5. Epub 2006 Aug 9. Pharm Res. 2006. PMID: 16952003
-
Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent.Proc Natl Acad Sci U S A. 2005 May 17;102(20):7292-6. doi: 10.1073/pnas.0500062102. Epub 2005 May 3. Proc Natl Acad Sci U S A. 2005. PMID: 15870205 Free PMC article.
-
Requirement for the immunoglobulin-like domain of granulocyte colony-stimulating factor receptor in formation of a 2:1 receptor-ligand complex.J Biol Chem. 1995 Oct 27;270(43):25928-34. doi: 10.1074/jbc.270.43.25928. J Biol Chem. 1995. PMID: 7592781
-
Fusion protein linkers: property, design and functionality.Adv Drug Deliv Rev. 2013 Oct;65(10):1357-69. doi: 10.1016/j.addr.2012.09.039. Epub 2012 Sep 29. Adv Drug Deliv Rev. 2013. PMID: 23026637 Free PMC article. Review.
-
Brain iron homeostasis.Dan Med Bull. 2002 Nov;49(4):279-301. Dan Med Bull. 2002. PMID: 12553165 Review.
Cited by
-
Research Advances in Fusion Protein-Based Drugs for Diabetes Treatment.Diabetes Metab Syndr Obes. 2024 Jan 23;17:343-362. doi: 10.2147/DMSO.S421527. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 38288338 Free PMC article. Review.
-
Tissue barriers and novel approaches to achieve hepatoselectivity of subcutaneously-injected insulin therapeutics.Tissue Barriers. 2016 Mar 4;4(2):e1156804. doi: 10.1080/21688370.2016.1156804. eCollection 2016 Apr-Jun. Tissue Barriers. 2016. PMID: 27358753 Free PMC article. Review.
-
Design an Efficient Multi-Epitope Peptide Vaccine Candidate Against SARS-CoV-2: An in silico Analysis.Infect Drug Resist. 2020 Aug 25;13:3007-3022. doi: 10.2147/IDR.S264573. eCollection 2020. Infect Drug Resist. 2020. PMID: 32943888 Free PMC article.
-
Enhanced insulin receptor interaction by a bifunctional insulin-transferrin fusion protein: an approach to overcome insulin resistance.Sci Rep. 2020 May 7;10(1):7724. doi: 10.1038/s41598-020-64731-9. Sci Rep. 2020. PMID: 32382087 Free PMC article.
-
Receptor-mediated activation of a proinsulin-transferrin fusion protein in hepatoma cells.J Control Release. 2011 Nov 7;155(3):386-92. doi: 10.1016/j.jconrel.2011.06.029. Epub 2011 Jul 2. J Control Release. 2011. PMID: 21756950 Free PMC article.
References
-
- Leader B, Baca Q, Golan D. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7:21–39. - PubMed
-
- Duttaroy A, Kanakaraj P, Osborn B, Schneider H, Pickeral O, Chen C, Zhang G, Kaithamana S, Singh M, Schulingkamp R, Crossan D, Bock J, Kaufman T, Reavey P, Carey-Barber M, Krishnan S, Garcia A, Murphy K, Siskind J, McLean M, Cheng S, Ruben S, Birse C, Blondel O. Development of a long-acting insulin analog using albumin fusion technology. Diabetes. 2005;54:251–8. - PubMed
-
- Osborn B, Olsen H, Nardelli B, Murray J, Zhou J, Garcia A, Moody G, Zaritskaya L, Sung C. Pharmacokinetic and pharmacodynamic studies of a human serum albumin-interferon-alpha fusion protein in cynomolgus monkeys. J Pharmacol Exp Ther. 2002;303:540–8. - PubMed
-
- Müller N, Schneider B, Pfizenmaier K, Wajant H. Superior serum half life of albumin tagged TNF ligands. Biochem Biophys Res Commun. 2010;396:793–9. - PubMed
-
- Peters R, Low S, Kamphaus G, Dumont J, Amari J, Lu Q, Zarbis-Papastoitsis G, Reidy T, Merricks E, Nichols T, Bitonti A. Prolonged activity of factor IX as a monomeric Fc fusion protein. Blood. 2010;115:2057–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

