Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth
- PMID: 21316604
- PMCID: PMC3060401
- DOI: 10.1016/j.ccr.2011.01.020
Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth
Abstract
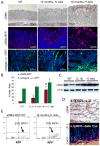
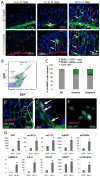
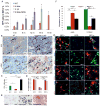
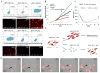
Carcinoma-associated fibroblasts (CAFs) that express α-smooth muscle actin (αSMA) contribute to cancer progression, but their precise origin and role are unclear. Using mouse models of inflammation-induced gastric cancer, we show that at least 20% of CAFs originate from bone marrow (BM) and derive from mesenchymal stem cells (MSCs). αSMA+ myofibroblasts (MFs) are niche cells normally present in BM and increase markedly during cancer progression. MSC-derived CAFs that are recruited to the dysplastic stomach express IL-6, Wnt5α and BMP4, show DNA hypomethylation, and promote tumor growth. Moreover, CAFs are generated from MSCs and are recruited to the tumor in a TGF-β- and SDF-1α-dependent manner. Therefore, carcinogenesis involves expansion and relocation of BM-niche cells to the tumor to create a niche to sustain cancer progression.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
CXCR4/TGF-β1 mediated self-differentiation of human mesenchymal stem cells to carcinoma-associated fibroblasts and promoted colorectal carcinoma development.Cancer Biol Ther. 2020;21(3):248-257. doi: 10.1080/15384047.2019.1685156. Epub 2019 Dec 10. Cancer Biol Ther. 2020. PMID: 31818187 Free PMC article.
-
Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors.Gut. 2013 Feb;62(2):192-200. doi: 10.1136/gutjnl-2011-301824. Epub 2012 Feb 23. Gut. 2013. PMID: 22362916 Free PMC article.
-
Bone marrow mesenchymal stem cells in microenvironment transform into cancer-associated fibroblasts to promote the progression of B-cell acute lymphoblastic leukemia.Biomed Pharmacother. 2020 Oct;130:110610. doi: 10.1016/j.biopha.2020.110610. Epub 2020 Aug 12. Biomed Pharmacother. 2020. PMID: 34321159
-
GRP78 secreted by tumor cells stimulates differentiation of bone marrow mesenchymal stem cells to cancer-associated fibroblasts.Biochem Biophys Res Commun. 2013 Nov 1;440(4):558-63. doi: 10.1016/j.bbrc.2013.09.108. Epub 2013 Oct 7. Biochem Biophys Res Commun. 2013. PMID: 24113381
-
Bone marrow-derived mesenchymal stem cells promote Helicobacter pylori-associated gastric cancer progression by secreting thrombospondin-2.Cell Prolif. 2021 Oct;54(10):e13114. doi: 10.1111/cpr.13114. Epub 2021 Aug 25. Cell Prolif. 2021. PMID: 34435402 Free PMC article.
Cited by
-
Wnt Inhibition Sensitizes PD-L1 Blockade Therapy by Overcoming Bone Marrow-Derived Myofibroblasts-Mediated Immune Resistance in Tumors.Front Immunol. 2021 Mar 15;12:619209. doi: 10.3389/fimmu.2021.619209. eCollection 2021. Front Immunol. 2021. PMID: 33790893 Free PMC article.
-
Bone marrow-derived myofibroblasts are the providers of pro-invasive matrix metalloproteinase 13 in primary tumor.Neoplasia. 2012 Oct;14(10):943-51. doi: 10.1593/neo.121092. Neoplasia. 2012. PMID: 23097628 Free PMC article.
-
The TLR7 agonist Imiquimod promote the immunogenicity of mesenchymal stem cells.Biol Res. 2015 Jan 17;48(1):6. doi: 10.1186/0717-6287-48-6. Biol Res. 2015. PMID: 25654296 Free PMC article.
-
Molecular pathways: involving microenvironment damage responses in cancer therapy resistance.Clin Cancer Res. 2012 Aug 1;18(15):4019-25. doi: 10.1158/1078-0432.CCR-11-0768. Epub 2012 May 22. Clin Cancer Res. 2012. PMID: 22619305 Free PMC article.
-
Extracellular vesicles from bone marrow mesenchymal stem/stromal cells transport tumor regulatory microRNA, proteins, and metabolites.Onco_target. 2015 Mar 10;6(7):4953-67. doi: 10.18632/onco_target.3211. Onco_target. 2015. PMID: 25669974 Free PMC article.
References
-
- Allinen M, Beroukhim R, Cai L, Brennan C, Lahti-Domenici J, Huang H, Porter D, Hu M, Chin L, Richardson A, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. - PubMed
-
- Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–4351. - PMC - PubMed
-
- Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. J Pathol. 2009;217:307–317. - PubMed
-
- Direkze NC, Forbes SJ, Brittan M, Hunt T, Jeffery R, Preston SL, Poulsom R, Hodivala-Dilke K, Alison MR, Wright NA. Multiple organ engraftment by bone-marrow-derived myofibroblasts and fibroblasts in bone-marrow-transplanted mice. Stem Cells. 2003;21:514–520. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

