CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells
- PMID: 21506999
- PMCID: PMC3112344
- DOI: 10.1111/j.1365-2567.2011.03446.x
CD11b and CD27 reflect distinct population and functional specialization in human natural killer cells
Abstract
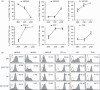
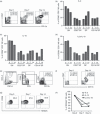
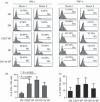
The identification of developmental stages in natural killer (NK) cells, especially in human NK cells, has lagged for decades. We characterize four novel populations defined by CD11b and CD27, which can represent the distinct stages of human NK cells from different tissues. Nearly all NK cells from peripheral blood are CD11b(+) CD27(-) populations whereas NK cells from cord blood have CD11b(+) CD27(-) and CD11b(+) CD27(+) populations. Interestingly, we have found large CD11b(-) CD27(-) populations of NK cells from deciduas. We also demonstrate that each population could be characterized by unique functional and phenotypic attributes. CD11b(-) CD27(-) NK cells display an immature phenotype and potential for differentiation. CD11b(-) CD27(+) and CD11b(+) CD27(+) NK cells show the best ability to secrete cytokines. CD11b(+) CD27(-) NK cells exhibit high cytolytic function. We demonstrate that human NK cells at different developmental stages have special functions and describe a new model of human NK cell differentiation.
© 2011 The Authors. Immunology © 2011 Blackwell Publishing Ltd.
Figures



Similar articles
-
Subsets of human natural killer cells and their regulatory effects.Immunology. 2014 Apr;141(4):483-9. doi: 10.1111/imm.12224. Immunology. 2014. PMID: 24303897 Free PMC article. Review.
-
Murine peripheral NK-cell populations originate from site-specific immature NK cells more than from BM-derived NK cells.Eur J Immunol. 2016 May;46(5):1258-70. doi: 10.1002/eji.201545847. Epub 2016 Mar 31. Eur J Immunol. 2016. PMID: 26919267
-
CD27-deficient mice show normal NK-cell differentiation but impaired function upon stimulation.Immunol Cell Biol. 2011 Oct;89(7):803-11. doi: 10.1038/icb.2010.171. Epub 2011 Feb 1. Immunol Cell Biol. 2011. PMID: 21283110
-
Monocytes control natural killer cell differentiation to effector phenotypes.Blood. 2011 Apr 28;117(17):4511-8. doi: 10.1182/blood-2010-10-312264. Epub 2011 Mar 9. Blood. 2011. PMID: 21389319
-
Functional subsets of mouse natural killer cells.Immunol Rev. 2006 Dec;214:47-55. doi: 10.1111/j.1600-065X.2006.00454.x. Immunol Rev. 2006. PMID: 17100875 Review.
Cited by
-
The Crosstalk Between Tumor Cells and the Immune Microenvironment in Breast Cancer: Implications for Immunotherapy.Front Oncol. 2021 Mar 11;11:610303. doi: 10.3389/fonc.2021.610303. eCollection 2021. Front Oncol. 2021. PMID: 33777750 Free PMC article. Review.
-
Primary cutaneous undifferentiated round cell tumor with concurrent polymyositis in a dog.Can Vet J. 2012 May;53(5):549-53. Can Vet J. 2012. PMID: 23115370 Free PMC article.
-
The role of CD56bright NK cells in neurodegenerative disorders.J Neuroinflammation. 2024 Feb 13;21(1):48. doi: 10.1186/s12974-024-03040-8. J Neuroinflammation. 2024. PMID: 38350967 Free PMC article. Review.
-
Three macrophage subsets are identified in the uterus during early human pregnancy.Cell Mol Immunol. 2018 Dec;15(12):1027-1037. doi: 10.1038/s41423-018-0008-0. Epub 2018 Apr 4. Cell Mol Immunol. 2018. PMID: 29618777 Free PMC article.
-
Deciphering Natural Killer Cell Cytotoxicity Against Medulloblastoma in vitro and in vivo: Implications for Immunotherapy.Immuno_targets Ther. 2024 Jun 26;13:319-333. doi: 10.2147/ITT.S458278. eCollection 2024. Immuno_targets Ther. 2024. PMID: 38948503 Free PMC article.
References
-
- Galy A, Travis M, Cen DZ, Chen B. Human T-cells, B-cells, natural-killer, and dendritic cells arise from a common bone-marrow progenitor-cell subset. Immunity. 1995;3:459–73. - PubMed
-
- Miller JS, Alley KA, Mcglave P. Differentiation of natural-killer (Nk) cells from human primitive marrow progenitors in a stroma-based long-term culture system – identification of a Cd34+ 7+ Nk progenitor. Blood. 1994;83:2594–601. - PubMed
-
- Shibuya A, Kojima H, Shibuya K, Nagayoshi K, Nagasawa T, Nakauchi H. Enrichment of interleukin-2-responsive natural-killer progenitors in human bone-marrow. Blood. 1993;81:1819–26. - PubMed
-
- Freud AG, Becknell B, Roychowdhury S, et al. A novel human CD34+ subset that constitutively expresses the high affinity interleukin-2 receptor traffics to lymph nodes and differentiates into CD56Bright natural killer cells. Blood. 2004;104:93a–93a.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

