Efficacy of everolimus, a novel mTOR inhibitor, against basal-like triple-negative breast cancer cells
- PMID: 22703543
- PMCID: PMC7659327
- DOI: 10.1111/j.1349-7006.2012.02359.x
Efficacy of everolimus, a novel mTOR inhibitor, against basal-like triple-negative breast cancer cells
Abstract
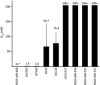
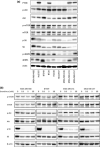
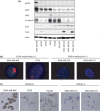
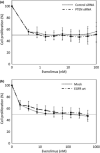
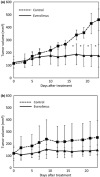
Patients with triple-negative breast cancers (TNBCs) typically have a poor prognosis because such cancers have no effective therapeutic _targets, such as estrogen receptors for endocrine therapy or human epidermal growth factor receptor 2 (HER2) receptors for anti-HER2 therapy. As the phosphatidylinositol 3' kinase (PI3K)/Akt/mammalian _target of rapamycin (mTOR) cascade is activated in TNBCs, mTOR is a potential molecular _target for anticancer therapy. In this study, we investigated the antitumor activities of everolimus, an oral mTOR inhibitor, in nine TNBC cell lines. Everolimus effectively inhibited cell growth at concentrations under 100 nM (IC(50)) in five cell lines and even in the 1-nM range in three of the five cell lines. To identify specific characteristics that could be used as predictive markers of efficacy, we evaluated the expressions of proteins in the mTOR cascade, basal markers, and cancer stem cell markers using western blotting, fluorescent in situ hybridization (FISH), or immunohistochemistry. All five of the sensitive cell lines were categorized as a basal-like subtype positive for either epidermal growth factor receptor (EGFR) or CK5/6, although resistant cell lines were not of this subtype and tended to exhibit the characteristics of cancer stem cells, with decreased E-cadherin and the increased expression of Snail or Twist. In vivo assays demonstrated antitumor activity in a mouse xenograft model of basal-like breast cancer, rather than non-basal breast cancer. These results suggest that everolimus has favorable activity against basal-like subtypes of TNBCs. Epidermal growth factor receptor and CK5/6 are positive predictive markers of the TNBC response to everolimus, while cancer stem cell markers are negative predictive markers.
© 2012 Japanese Cancer Association.
Figures
Similar articles
-
Combined _targeting of mTOR and AKT is an effective strategy for basal-like breast cancer in patient-derived xenograft models.Mol Cancer Ther. 2013 Aug;12(8):1665-75. doi: 10.1158/1535-7163.MCT-13-0159. Epub 2013 May 20. Mol Cancer Ther. 2013. PMID: 23689832 Free PMC article.
-
Overcoming endocrine resistance due to reduced PTEN levels in estrogen receptor-positive breast cancer by co-_targeting mammalian _target of rapamycin, protein kinase B, or mitogen-activated protein kinase kinase.Breast Cancer Res. 2014 Sep 11;16(5):430. doi: 10.1186/s13058-014-0430-x. Breast Cancer Res. 2014. PMID: 25212826 Free PMC article.
-
Effectiveness and molecular interactions of the clinically active mTORC1 inhibitor everolimus in combination with tamoxifen or letrozole in vitro and in vivo.Breast Cancer Res. 2012 Oct 17;14(5):R132. doi: 10.1186/bcr3330. Breast Cancer Res. 2012. PMID: 23075476 Free PMC article.
-
Everolimus: _targeted therapy on the horizon for the treatment of breast cancer.Pharmacotherapy. 2012 Apr;32(4):383-96. doi: 10.1002/j.1875-9114.2012.01084.x. Pharmacotherapy. 2012. PMID: 22461124 Review.
-
mTOR inhibitors in advanced breast cancer: ready for prime time?Cancer Treat Rev. 2013 Nov;39(7):742-52. doi: 10.1016/j.ctrv.2013.02.005. Epub 2013 Apr 1. Cancer Treat Rev. 2013. PMID: 23557794 Review.
Cited by
-
The bromodomain inhibitor OTX015 (MK-8628) exerts anti-tumor activity in triple-negative breast cancer models as single agent and in combination with everolimus.Onco_target. 2017 Jan 31;8(5):7598-7613. doi: 10.18632/onco_target.13814. Onco_target. 2017. PMID: 27935867 Free PMC article.
-
Selectively _targeting Breast Cancer Stem Cells by 8-Quinolinol and Niclosamide.Int J Mol Sci. 2022 Oct 4;23(19):11760. doi: 10.3390/ijms231911760. Int J Mol Sci. 2022. PMID: 36233074 Free PMC article.
-
Therapies for triple negative breast cancer.Expert Opin Pharmacother. 2015 May;16(7):983-98. doi: 10.1517/14656566.2015.1032246. Expert Opin Pharmacother. 2015. PMID: 25881743 Free PMC article. Review.
-
DIF-1 inhibits growth and metastasis of triple-negative breast cancer through AMPK-mediated inhibition of the mTORC1-S6K signaling pathway.Oncogene. 2021 Sep;40(37):5579-5589. doi: 10.1038/s41388-021-01958-4. Epub 2021 Jul 24. Oncogene. 2021. PMID: 34304250
-
Triple-negative breast cancer: new treatment strategies in the era of precision medicine.Sci China Life Sci. 2021 Mar;64(3):372-388. doi: 10.1007/s11427-020-1714-8. Epub 2020 Aug 11. Sci China Life Sci. 2021. PMID: 32803712 Review.
References
-
- Dent R, Trudeau M, Pritchard KI et al Triple‐negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res 2007; 13: 4429–34. - PubMed
-
- Liedtke C, Mazouni C, Hess KR et al Response to neoadjuvant therapy and long‐term survival in patients with triple‐negative breast cancer. J Clin ncol 2008; 26: 1275–81. - PubMed
-
- Thike AA, Cheok PY, Jara‐Lazaro AR, Tan B, Tan P, Tan PH. Triple‐negative breast cancer: clinicopathological characteristics and relationship with basal‐like breast cancer. Mod Pathol 2010; 23: 123–33. - PubMed
-
- Colleoni M, Cole BF, Viale G et al Classical cyclophosphamide, methotrexate, and fluorouracil chemotherapy is more effective in triple‐negative, node‐negative breast cancer: results from two randomized trials of adjuvant chemoendocrine therapy for node‐negative breast cancer. J Clin Oncol 2010; 28: 2966–73. - PMC - PubMed
-
- Foulkes WD, Stefansson IM, Chappuis PO et al Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J Natl Cancer Inst 2003; 95: 1482–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

