Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae
- PMID: 22952924
- PMCID: PMC3432075
- DOI: 10.1371/journal.pone.0044192
Calcineurin is required for pseudohyphal growth, virulence, and drug resistance in Candida lusitaniae
Abstract
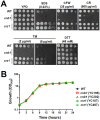
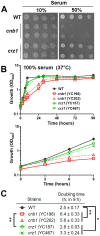
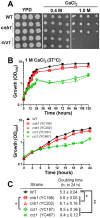
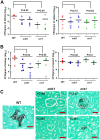
Candida lusitaniae is an emerging fungal pathogen that infects immunocompromised patients including HIV/AIDS, cancer, and neonatal pediatric patients. Though less prevalent than other Candida species, C. lusitaniae is unique in its ability to develop resistance to amphotericin B. We investigated the role of the calcium-activated protein phosphatase calcineurin in several virulence attributes of C. lusitaniae including pseudohyphal growth, serum survival, and growth at 37°C. We found that calcineurin and Crz1, a C. albicans Crz1 homolog acting as a downstream _target of calcineurin, are required for C. lusitaniae pseudohyphal growth, a process for which the underlying mechanism remains largely unknown in C. lusitaniae but hyphal growth is fundamental to C. albicans virulence. We demonstrate that calcineurin is required for cell wall integrity, ER stress response, optimal growth in serum, virulence in a murine systemic infection model, and antifungal drug tolerance in C. lusitaniae. To further examine the potential of _targeting the calcineurin signaling cascade for antifungal drug development, we examined the activity of a calcineurin inhibitor FK506 in combination with caspofungin against echinocandin resistant C. lusitaniae clinical isolates. Broth microdilution and drug disk diffusion assays demonstrate that FK506 has synergistic fungicidal activity with caspofungin against echinocandin resistant isolates. Our findings reveal that pseudohyphal growth is controlled by the calcineurin signaling cascade, and highlight the potential use of calcineurin inhibitors and caspofungin for emerging drug-resistant C. lusitaniae infections.
Conflict of interest statement
Figures



Similar articles
-
Calcineurin controls hyphal growth, virulence, and drug tolerance of Candida tropicalis.Eukaryot Cell. 2014 Jul;13(7):844-54. doi: 10.1128/EC.00302-13. Epub 2014 Jan 17. Eukaryot Cell. 2014. PMID: 24442892 Free PMC article.
-
Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis.Eukaryot Cell. 2011 Jun;10(6):803-19. doi: 10.1128/EC.00310-10. Epub 2011 Apr 29. Eukaryot Cell. 2011. PMID: 21531874 Free PMC article.
-
Elevated chitin content reduces the susceptibility of Candida species to caspofungin.Antimicrob Agents Chemother. 2013 Jan;57(1):146-54. doi: 10.1128/AAC.01486-12. Epub 2012 Oct 22. Antimicrob Agents Chemother. 2013. PMID: 23089748 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
Calcineurin signaling: lessons from Candida species.FEMS Yeast Res. 2015 Jun;15(4):fov016. doi: 10.1093/femsyr/fov016. Epub 2015 Apr 15. FEMS Yeast Res. 2015. PMID: 25878052 Review.
Cited by
-
Calcineurin controls hyphal growth, virulence, and drug tolerance of Candida tropicalis.Eukaryot Cell. 2014 Jul;13(7):844-54. doi: 10.1128/EC.00302-13. Epub 2014 Jan 17. Eukaryot Cell. 2014. PMID: 24442892 Free PMC article.
-
Updating Insights into the Regulatory Mechanisms of Calcineurin-Activated Transcription Factor Crz1 in Pathogenic Fungi.J Fungi (Basel). 2022 Oct 14;8(10):1082. doi: 10.3390/jof8101082. J Fungi (Basel). 2022. PMID: 36294647 Free PMC article. Review.
-
Posaconazole exhibits in vitro and in vivo synergistic antifungal activity with caspofungin or FK506 against Candida albicans.PLoS One. 2013;8(3):e57672. doi: 10.1371/journal.pone.0057672. Epub 2013 Mar 5. PLoS One. 2013. PMID: 23472097 Free PMC article.
-
Calcineurin in fungal virulence and drug resistance: Prospects for harnessing _targeted inhibition of calcineurin for an antifungal therapeutic approach.Virulence. 2017 Feb 17;8(2):186-197. doi: 10.1080/21505594.2016.1201250. Epub 2016 Jun 20. Virulence. 2017. PMID: 27325145 Free PMC article. Review.
-
Genetic analysis of Hsp90 function in Cryptococcus neoformans highlights key roles in stress tolerance and virulence.Genetics. 2022 Jan 4;220(1):iyab164. doi: 10.1093/genetics/iyab164. Genetics. 2022. PMID: 34849848 Free PMC article.
References
-
- Atkinson BJ, Lewis RE, Kontoyiannis DP (2008) Candida lusitaniae fungemia in cancer patients: risk factors for amphotericin B failure and outcome. Med Mycol 46: 541–546. - PubMed
-
- Hawkins JL, Baddour LM (2003) Candida lusitaniae infections in the era of fluconazole availability. Clin Infect Dis 36: e14–18. - PubMed
-
- Minari A, Hachem R, Raad I (2001) Candida lusitaniae: a cause of breakthrough fungemia in cancer patients. Clin Infect Dis 32: 186–190. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

