Sonic hedgehog promotes autophagy of vascular smooth muscle cells
- PMID: 23023870
- PMCID: PMC3532542
- DOI: 10.1152/ajpheart.00160.2012
Sonic hedgehog promotes autophagy of vascular smooth muscle cells
Abstract
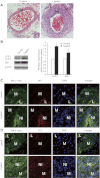
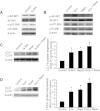
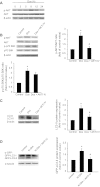
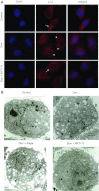
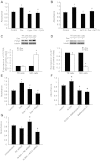
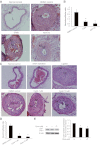
Sonic hedgehog (Shh) is a morphogen critically involved in development that is reexpressed in atherosclerotic lesions. It also stimulates proliferation of vascular smooth muscle cells (SMCs). Autophagy in vascular SMCs is known to promote SMC survival and increase plaque stability. The aim of this study was to investigate whether Shh induces autophagy of vascular SMCs. Our study showed that both Shh protein and microtubule-associated protein 1 light chain 3 (LC3)-II were increased in SMCs within neointimal lesions of mouse common carotid arteries. In cultured mouse aortic SMCs, recombinant mouse Shh stimulated LC3-II levels. Overexpression of wild-type mouse Shh through the tetracycline-regulated expression-inducible system in human aortic SMCs time-dependently increased the levels of LC3-II and also stimulated protein kinase B (AKT) phosphorylation. Pretreatment with AKT inhibitor IV (AKTI IV) inhibited AKT phosphorylation and the increase in LC3-II. Shh-induced autophagy was further confirmed by the formation of autophagosomes as detected by immunostaining and transmission electron microscopy, which was inhibited by AKTI IV. Shh further increased SMC LC3-II in the presence of bafilomycin A1, (2S,3S)-trans-epoxysuccinyl-L-leucylamido-3-methylbutane ethyl ester, and pepstatin A or siRNA for the autophagy-related gene 7 (ATG7). In addition, Shh induced SMC proliferation, which was inhibited not only by AKTI IV but also by cyclopamine, an inhibitor of Shh receptor. Inhibition of autophagy with 3-methyladenine (3-MA), bafilomycin A1, or ATG7 siRNA resulted in inhibition of cell proliferation. Treatment with 3-MA, AKTI IV, or cyclopamine inhibited neointima formation in mouse common carotid arteries. Taken together, our results have shown that Shh induces autophagy of vascular SMCs involving AKT activation, suggesting a role of autophagy in Shh-induced cellular responses.
Figures

Similar articles
-
Sonic hedgehog-dependent activation of adventitial fibroblasts promotes neointima formation.Cardiovasc Res. 2017 Nov 1;113(13):1653-1663. doi: 10.1093/cvr/cvx158. Cardiovasc Res. 2017. PMID: 29088375
-
Sonic hedgehog signaling induces vascular smooth muscle cell proliferation via induction of the G1 cyclin-retinoblastoma axis.Arterioscler Thromb Vasc Biol. 2010 Sep;30(9):1787-94. doi: 10.1161/ATVBAHA.110.208520. Arterioscler Thromb Vasc Biol. 2010. PMID: 20720195
-
Cortistatin inhibits migration and proliferation of human vascular smooth muscle cells and decreases neointimal formation on carotid artery ligation.Circ Res. 2013 May 24;112(11):1444-55. doi: 10.1161/CIRCRESAHA.112.300695. Epub 2013 Apr 17. Circ Res. 2013. PMID: 23595952
-
The role of vascular smooth muscle cell membrane-bound thrombomodulin in neointima formation.Atherosclerosis. 2019 Aug;287:54-63. doi: 10.1016/j.atherosclerosis.2019.05.019. Epub 2019 May 24. Atherosclerosis. 2019. PMID: 31212235
-
Metformin alleviates hyperglycemia-induced endothelial impairment by downregulating autophagy via the Hedgehog pathway.Autophagy. 2019 May;15(5):843-870. doi: 10.1080/15548627.2019.1569913. Epub 2019 Jan 27. Autophagy. 2019. PMID: 30653446 Free PMC article.
Cited by
-
Autophagy: an emerging therapeutic _target in vascular diseases.Br J Pharmacol. 2015 May;172(9):2167-78. doi: 10.1111/bph.13052. Epub 2015 Feb 27. Br J Pharmacol. 2015. PMID: 25537552 Free PMC article. Review.
-
Primary Cilium in Cancer Hallmarks.Int J Mol Sci. 2019 Mar 16;20(6):1336. doi: 10.3390/ijms20061336. Int J Mol Sci. 2019. PMID: 30884815 Free PMC article. Review.
-
Loss of Tctn3 causes neuronal apoptosis and neural tube defects in mice.Cell Death Dis. 2018 May 1;9(5):520. doi: 10.1038/s41419-018-0563-4. Cell Death Dis. 2018. PMID: 29725084 Free PMC article.
-
c-Ski inhibits autophagy of vascular smooth muscle cells induced by oxLDL and PDGF.PLoS One. 2014 Jun 2;9(6):e98902. doi: 10.1371/journal.pone.0098902. eCollection 2014. PLoS One. 2014. PMID: 24887307 Free PMC article.
-
Cilia in autophagy and cancer.Cilia. 2016 Feb 3;5:4. doi: 10.1186/s13630-016-0027-3. eCollection 2015. Cilia. 2016. PMID: 26848389 Free PMC article. Review.
References
-
- Bijlsma MF, Peppelenbosch MP, Spek CA. Hedgehog morphogen in cardiovascular disease. Circulation 114: 1985–1991, 2006 - PubMed
-
- Bijlsma MF, Spek CA. The Hedgehog morphogen in myocardial ischemia-reperfusion injury. Exp Biol Med (Maywood) 235: 447–454, 2010 - PubMed
-
- Byrd N, Becker S, Maye P, Narasimhaiah R, St-Jacques B, Zhang X, McMahon J, McMahon A, Grabel L. Hedgehog is required for murine yolk sac angiogenesis. Development 129: 361–372, 2002 - PubMed
-
- Byrd N, Grabel L. Hedgehog signaling in murine vasculogenesis and angiogenesis. Trends Cardiovasc Med 14: 308–313, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

