Celiac disease: prevalence, diagnosis, pathogenesis and treatment
- PMID: 23155333
- PMCID: PMC3496881
- DOI: 10.3748/wjg.v18.i42.6036
Celiac disease: prevalence, diagnosis, pathogenesis and treatment
Abstract
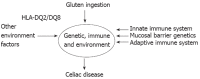
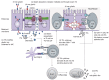
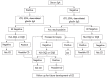
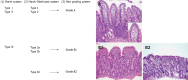
Celiac disease (CD) is one of the most common diseases, resulting from both environmental (gluten) and genetic factors [human leukocyte antigen (HLA) and non-HLA genes]. The prevalence of CD has been estimated to approximate 0.5%-1% in different parts of the world. However, the population with diabetes, autoimmune disorder or relatives of CD individuals have even higher risk for the development of CD, at least in part, because of shared HLA typing. Gliadin gains access to the basal surface of the epithelium, and interact directly with the immune system, via both trans- and para-cellular routes. From a diagnostic perspective, symptoms may be viewed as either "typical" or "atypical". In both positive serological screening results suggestive of CD, should lead to small bowel biopsy followed by a favourable clinical and serological response to the gluten-free diet (GFD) to confirm the diagnosis. Positive anti-tissue transglutaminase antibody or anti-endomysial antibody during the clinical course helps to confirm the diagnosis of CD because of their over 99% specificities when small bowel villous atrophy is present on biopsy. Currently, the only treatment available for CD individuals is a strict life-long GFD. A greater understanding of the pathogenesis of CD allows alternative future CD treatments to hydrolyse toxic gliadin peptide, prevent toxic gliadin peptide absorption, blockage of selective deamidation of specific glutamine residues by tissue, restore immune tolerance towards gluten, modulation of immune response to dietary gliadin, and restoration of intestinal architecture.
Keywords: Celiac disease; Demography; Diagnosis; Pathogenesis; Treatment.
Figures



Similar articles
-
[An analysis of clinical features of celiac disease patients in different ethnic].Zhonghua Nei Ke Za Zhi. 2016 Aug 1;55(8):613-8. doi: 10.3760/cma.j.issn.0578-1426.2016.08.009. Zhonghua Nei Ke Za Zhi. 2016. PMID: 27480555 Chinese.
-
Celiac disease: risk assessment, diagnosis, and monitoring.Mol Diagn Ther. 2008;12(5):289-98. doi: 10.1007/BF03256294. Mol Diagn Ther. 2008. PMID: 18803427 Review.
-
Coeliac disease.Paediatr Int Child Health. 2019 Feb;39(1):23-31. doi: 10.1080/20469047.2018.1504431. Epub 2018 Aug 13. Paediatr Int Child Health. 2019. PMID: 30099930 Review.
-
Prevalence of celiac disease and celiac autoimmunity in the Toba Native Amerindian community of Argentina.Can J Gastroenterol Hepatol. 2015 Nov-Dec;29(8):431-4. doi: 10.1155/2015/927458. Epub 2015 Jul 24. Can J Gastroenterol Hepatol. 2015. PMID: 26207618 Free PMC article.
-
Transglutaminase 2 and Transglutaminase 2 Autoantibodies in Celiac Disease: a Review.Clin Rev Allergy Immunol. 2019 Aug;57(1):23-38. doi: 10.1007/s12016-016-8557-4. Clin Rev Allergy Immunol. 2019. PMID: 27263022 Review.
Cited by
-
Determination of the Frequency of Celiac Disease in Patients Presenting With Chronic Diarrhea.Cureus. 2024 Jul 2;16(7):e63638. doi: 10.7759/cureus.63638. eCollection 2024 Jul. Cureus. 2024. PMID: 38983671 Free PMC article.
-
Impact of 2'-Fucosyllactose on Gut Microbiota Composition in Adults with Chronic Gastrointestinal Conditions: Batch Culture Fermentation Model and Pilot Clinical Trial Findings.Nutrients. 2021 Mar 14;13(3):938. doi: 10.3390/nu13030938. Nutrients. 2021. PMID: 33799455 Free PMC article.
-
Multidimensional Disadvantages of a Gluten-Free Diet in Celiac Disease: A Narrative Review.Nutrients. 2021 Feb 16;13(2):643. doi: 10.3390/nu13020643. Nutrients. 2021. PMID: 33669442 Free PMC article. Review.
-
Coeliac disease and postpartum depression: are they linked? A two-sample Mendelian randomization study.Front Psychiatry. 2024 Jul 19;15:1312117. doi: 10.3389/fpsyt.2024.1312117. eCollection 2024. Front Psychiatry. 2024. PMID: 39100855 Free PMC article.
-
Celiac Crisis: an unusual presentation of gluten-sensitive enteropathy.Autops Case Rep. 2018 Jul 30;8(3):e2018027. doi: 10.4322/acr.2018.027. eCollection 2018 Jul-Sep. Autops Case Rep. 2018. PMID: 30101133 Free PMC article.
References
-
- Rampertab SD, Pooran N, Brar P, Singh P, Green PH. Trends in the presentation of celiac disease. Am J Med. 2006;119:355.e9–355.14. - PubMed
-
- Mäki M, Kallonen K, Lähdeaho ML, Visakorpi JK. Changing pattern of childhood coeliac disease in Finland. Acta Paediatr Scand. 1988;77:408–412. - PubMed
-
- Tursi A, Brandimarte G, Giorgetti G, Gigliobianco A, Lombardi D, Gasbarrini G. Low prevalence of antigliadin and anti-endomysium antibodies in subclinical/silent celiac disease. Am J Gastroenterol. 2001;96:1507–1510. - PubMed
-
- Bai D, Brar P, Holleran S, Ramakrishnan R, Green PH. Effect of gender on the manifestations of celiac disease: evidence for greater malabsorption in men. Scand J Gastroenterol. 2005;40:183–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

