Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-β-catenin signaling
- PMID: 23299311
- PMCID: PMC3591802
- DOI: 10.1182/blood-2012-02-412890
Microenvironmental protection of CML stem and progenitor cells from tyrosine kinase inhibitors through N-cadherin and Wnt-β-catenin signaling
Abstract
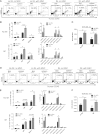
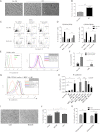
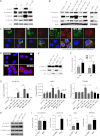
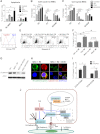
Tyrosine kinase inhibitors (TKIs) are highly effective in treatment of chronic myeloid leukemia (CML) but do not eliminate leukemia stem cells (LSCs), which remain a potential source of relapse. TKI treatment effectively inhibits BCR-ABL kinase activity in CML LSCs, suggesting that additional kinase-independent mechanisms contribute to LSC preservation. We investigated whether signals from the bone marrow (BM) microenvironment protect CML LSCs from TKI treatment. Coculture with human BM mesenchymal stromal cells (MSCs) significantly inhibited apoptosis and preserved CML stem/progenitor cells following TKI exposure, maintaining colony-forming ability and engraftment potential in immunodeficient mice. We found that the N-cadherin receptor plays an important role in MSC-mediated protection of CML progenitors from TKI. N-cadherin-mediated adhesion to MSCs was associated with increased cytoplasmic N-cadherin-β-catenin complex formation as well as enhanced β-catenin nuclear translocation and transcriptional activity. Increased exogenous Wnt-mediated β-catenin signaling played an important role in MSC-mediated protection of CML progenitors from TKI treatment. Our results reveal a close interplay between N-cadherin and the Wnt-β-catenin pathway in protecting CML LSCs during TKI treatment. Importantly, these results reveal novel mechanisms of resistance of CML LSCs to TKI treatment and suggest new _targets for treatment designed to eradicate residual LSCs in CML patients.
Figures


Similar articles
-
Anthelmintic Niclosamide Disrupts the Interplay of p65 and FOXM1/β-catenin and Eradicates Leukemia Stem Cells in Chronic Myelogenous Leukemia.Clin Cancer Res. 2017 Feb 1;23(3):789-803. doi: 10.1158/1078-0432.CCR-16-0226. Epub 2016 Aug 4. Clin Cancer Res. 2017. PMID: 27492973
-
A novel HDAC inhibitor chidamide combined with imatinib synergistically _targets tyrosine kinase inhibitor resistant chronic myeloid leukemia cells.Biomed Pharmacother. 2020 Sep;129:110390. doi: 10.1016/j.biopha.2020.110390. Epub 2020 Jun 17. Biomed Pharmacother. 2020. PMID: 32563150
-
Combined inhibition of β-catenin and Bcr-Abl synergistically _targets tyrosine kinase inhibitor-resistant blast crisis chronic myeloid leukemia blasts and progenitors in vitro and in vivo.Leukemia. 2017 Oct;31(10):2065-2074. doi: 10.1038/leu.2017.87. Epub 2017 Mar 21. Leukemia. 2017. PMID: 28321124 Free PMC article.
-
Druggable Biochemical Pathways and Potential Therapeutic Alternatives to _target Leukemic Stem Cells and Eliminate the Residual Disease in Chronic Myeloid Leukemia.Int J Mol Sci. 2019 Nov 10;20(22):5616. doi: 10.3390/ijms20225616. Int J Mol Sci. 2019. PMID: 31717629 Free PMC article. Review.
-
Preservation of Quiescent Chronic Myelogenous Leukemia Stem Cells by the Bone Marrow Microenvironment.Adv Exp Med Biol. 2018;1100:97-110. doi: 10.1007/978-3-319-97746-1_6. Adv Exp Med Biol. 2018. PMID: 30411262 Review.
Cited by
-
RNA modification in normal hematopoiesis and hematologic malignancies.MedComm (2020). 2024 Oct 23;5(11):e787. doi: 10.1002/mco2.787. eCollection 2024 Nov. MedComm (2020). 2024. PMID: 39445003 Free PMC article. Review.
-
14-3-3 Binding and Sumoylation Concur to the Down-Modulation of β-catenin Antagonist chibby 1 in Chronic Myeloid Leukemia.PLoS One. 2015 Jul 6;10(7):e0131074. doi: 10.1371/journal.pone.0131074. eCollection 2015. PLoS One. 2015. PMID: 26147002 Free PMC article.
-
High IL-7 levels in the bone marrow microenvironment mediate imatinib resistance and predict disease progression in chronic myeloid leukemia.Int J Hematol. 2016 Sep;104(3):358-67. doi: 10.1007/s12185-016-2028-9. Epub 2016 Jun 6. Int J Hematol. 2016. PMID: 27272942
-
Chronic myeloid leukemia: overview of new agents and comparative analysis.Curr Treat Options Oncol. 2013 Jun;14(2):127-43. doi: 10.1007/s11864-013-0234-8. Curr Treat Options Oncol. 2013. PMID: 23572291 Review.
-
Fibroblast activation protein α in tumor microenvironment: recent progression and implications (review).Mol Med Rep. 2015 May;11(5):3203-11. doi: 10.3892/mmr.2015.3197. Epub 2015 Jan 14. Mol Med Rep. 2015. PMID: 25593080 Free PMC article. Review.
References
-
- Sawyers CL. Chronic myeloid leukemia. N Engl J Med. 1999;340(17):1330–1340. - PubMed
-
- Druker BJ, Guilhot F, O’Brien SG, et al. IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. - PubMed
-
- Quintás-Cardama A, Kantarjian H, Cortes J. Imatinib and beyond—exploring the full potential of _targeted therapy for CML. Nat Rev Clin Oncol. 2009;6(9):535–543. - PubMed
-
- Mahon FX, Réa D, Guilhot J, et al. Intergroupe Français des Leucémies Myéloïdes Chroniques. Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11(11):1029–1035. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

