Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms
- PMID: 23314962
- PMCID: PMC3629765
- DOI: 10.1128/EC.00287-12
Extracellular DNA release acts as an antifungal resistance mechanism in mature Aspergillus fumigatus biofilms
Abstract
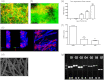
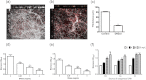
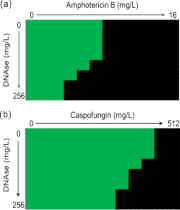
Aspergillus fumigatus has been shown to form biofilms that are associated with adaptive antifungal resistance mechanisms. These include multidrug efflux pumps, heat shock proteins, and extracellular matrix (ECM). ECM is a key structural and protective component of microbial biofilms and in bacteria has been shown to contain extracellular DNA (eDNA). We therefore hypothesized that A. fumigatus biofilms also possess eDNA as part of the ECM, conferring a functional role. Fluorescence microscopy and quantitative PCR analyses demonstrated the presence of eDNA, which was released phase dependently (8 < 12 < 24 < 48 h). Random amplification of polymorphic DNA (RAPD) PCR showed that eDNA was identical to genomic DNA. Biofilm architectural integrity was destabilized by DNase treatment. Biochemical and transcriptional analyses showed that chitinase activity and mRNA levels of chitinase, a marker of autolysis, were significantly upregulated as the biofilm matured and that inhibition of chitinases affected biofilm growth and stability, indicating mechanistically that autolysis was possibly involved. Finally, using checkerboard assays, it was shown that combinational treatment of biofilms with DNase plus amphotericin B and caspofungin significantly improved antifungal susceptibility. Collectively, these data show that eDNA is an important structural component of A. fumigatus ECM that is released through autolysis, which is important for protection from environmental stresses, including antifungal therapy.
Figures

Similar articles
-
Prior in vitro exposure to voriconazole confers resistance to amphotericin B in Aspergillus fumigatus biofilms.Int J Antimicrob Agents. 2015 Sep;46(3):342-5. doi: 10.1016/j.ijantimicag.2015.03.006. Epub 2015 Apr 24. Int J Antimicrob Agents. 2015. PMID: 25979638
-
Extracellular DNA release confers heterogeneity in Candida albicans biofilm formation.BMC Microbiol. 2014 Dec 5;14:303. doi: 10.1186/s12866-014-0303-6. BMC Microbiol. 2014. PMID: 25476750 Free PMC article.
-
Addition of DNase improves the in vitro activity of antifungal drugs against Candida albicans biofilms.Mycoses. 2012 Jan;55(1):80-5. doi: 10.1111/j.1439-0507.2011.02047.x. Epub 2011 Jun 12. Mycoses. 2012. PMID: 21668524 Free PMC article.
-
Aspergillus fumigatus biofilms: Toward understanding how growth as a multicellular network increases antifungal resistance and disease progression.PLoS Pathog. 2021 Aug 26;17(8):e1009794. doi: 10.1371/journal.ppat.1009794. eCollection 2021 Aug. PLoS Pathog. 2021. PMID: 34437655 Free PMC article. Review.
-
The characteristics of Aspergillus fumigatus mycetoma development: is this a biofilm?Med Mycol. 2009;47 Suppl 1:S120-6. doi: 10.1080/13693780802238834. Epub 2008 Jul 24. Med Mycol. 2009. PMID: 18654926 Review.
Cited by
-
Biofilms 2012: new discoveries and significant wrinkles in a dynamic field.J Bacteriol. 2013 Jul;195(13):2947-58. doi: 10.1128/JB.00239-13. Epub 2013 Apr 26. J Bacteriol. 2013. PMID: 23625847 Free PMC article. Review.
-
Interplay between biofilm microenvironment and pathogenicity of Pseudomonas aeruginosa in cystic fibrosis lung chronic infection.Biofilm. 2022 Oct 22;4:100089. doi: 10.1016/j.bioflm.2022.100089. eCollection 2022 Dec. Biofilm. 2022. PMID: 36324525 Free PMC article. Review.
-
Harnessing Bacterial Signals for Suppression of Biofilm Formation in the Nosocomial Fungal Pathogen Aspergillus fumigatus.Front Microbiol. 2016 Dec 22;7:2074. doi: 10.3389/fmicb.2016.02074. eCollection 2016. Front Microbiol. 2016. PMID: 28066389 Free PMC article.
-
Fungal biofilm architecture produces hypoxic microenvironments that drive antifungal resistance.Proc Natl Acad Sci U S A. 2020 Sep 8;117(36):22473-22483. doi: 10.1073/pnas.2003700117. Epub 2020 Aug 26. Proc Natl Acad Sci U S A. 2020. PMID: 32848055 Free PMC article.
-
Fluconazole impacts the extracellular matrix of fluconazole-susceptible and -resistant Candida albicans and Candida glabrata biofilms.J Oral Microbiol. 2018 Jun 4;10(1):1476644. doi: 10.1080/20002297.2018.1476644. eCollection 2018. J Oral Microbiol. 2018. PMID: 29887974 Free PMC article.
References
-
- Muller FM, Seidler M, Beauvais A. 2011. Aspergillus fumigatus biofilms in the clinical setting. Med. Mycol. 49(Suppl 1): S96–S100 - PubMed
-
- Ramage G, Rajendran R, Gutierrez-Correa M, Jones B, Williams C. 2011. Aspergillus biofilms: clinical and industrial significance. FEMS Microbiol. Lett. 324: 89–97 - PubMed
-
- Escande W, Fayad G, Modine T, Verbrugge E, Koussa M, Senneville E, Leroy O. 2011. Culture of a prosthetic valve excised for streptococcal endocarditis positive for Aspergillus fumigatus 20 years after a previous fumigatus endocarditis. Ann. Thorac. Surg. 91: e92–e93 - PubMed
-
- Jeloka TK, Shrividya S, Wagholikar G. 2011. Catheter outflow obstruction due to an aspergilloma. Perit. Dial. Int. 31: 211–212 - PubMed
-
- Langer P, Kassim RA, Macari GS, Saleh KJ. 2003. Aspergillus infection after total knee arthroplasty. Am. J. Orthop. 32: 402–404 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

