Phosphatidic acid and lipid-sensing by mTOR
- PMID: 23507202
- PMCID: PMC3669661
- DOI: 10.1016/j.tem.2013.02.003
Phosphatidic acid and lipid-sensing by mTOR
Abstract
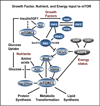
Mammalian _target of rapamycin (mTOR) has been implicated as a sensor of nutrient sufficiency for dividing cells and is activated by essential amino acids and glucose. However, cells also require lipids for membrane biosynthesis. A central metabolite in the synthesis of membrane phospholipids is phosphatidic acid (PA), which is required for the stability and activity of mTOR complexes. Although PA is commonly generated by the phospholipase D-catalyzed hydrolysis of phosphatidylcholine, PA is also generated by diacylglycerol kinases and lysophosphatidic acid acyltransferases, which are at the center of phospholipid biosynthesis. It is proposed that the responsiveness of mTOR/TOR to PA evolved as a means for sensing lipid precursors for membrane biosynthesis prior to doubling the mass of a cell and dividing.
Copyright © 2013 Elsevier Ltd. All rights reserved.
Figures


Similar articles
-
Phospholipase D and the maintenance of phosphatidic acid levels for regulation of mammalian _target of rapamycin (mTOR).J Biol Chem. 2014 Aug 15;289(33):22583-22588. doi: 10.1074/jbc.R114.566091. Epub 2014 Jul 2. J Biol Chem. 2014. PMID: 24990952 Free PMC article. Review.
-
Reciprocal regulation of AMP-activated protein kinase and phospholipase D.J Biol Chem. 2015 Mar 13;290(11):6986-93. doi: 10.1074/jbc.M114.622571. Epub 2015 Jan 29. J Biol Chem. 2015. PMID: 25632961 Free PMC article.
-
The role of diacylglycerol kinase ζ and phosphatidic acid in the mechanical activation of mammalian _target of rapamycin (mTOR) signaling and skeletal muscle hypertrophy.J Biol Chem. 2014 Jan 17;289(3):1551-63. doi: 10.1074/jbc.M113.531392. Epub 2013 Dec 3. J Biol Chem. 2014. PMID: 24302719 Free PMC article.
-
Inhibited insulin signaling in mouse hepatocytes is associated with increased phosphatidic acid but not diacylglycerol.J Biol Chem. 2015 Feb 6;290(6):3519-28. doi: 10.1074/jbc.M114.602789. Epub 2014 Dec 15. J Biol Chem. 2015. PMID: 25512376 Free PMC article.
-
Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells.Biochim Biophys Acta. 2009 Sep;1791(9):949-55. doi: 10.1016/j.bbalip.2009.02.009. Epub 2009 Mar 2. Biochim Biophys Acta. 2009. PMID: 19264150 Free PMC article. Review.
Cited by
-
Effects of Different Levels of Carbohydrates on Growth Performance, Hepatic and Intestinal Health, and Intestinal Microflora of Juvenile Pikeperch (Sander lucioperca).Aquac Nutr. 2024 Aug 9;2024:8450154. doi: 10.1155/2024/8450154. eCollection 2024. Aquac Nutr. 2024. PMID: 39555508 Free PMC article.
-
Phosphatidic acid directly activates mTOR and then regulates SREBP to promote ganoderic acid biosynthesis under heat stress in Ganoderma lingzhi.Commun Biol. 2024 Nov 13;7(1):1503. doi: 10.1038/s42003-024-07225-y. Commun Biol. 2024. PMID: 39537975 Free PMC article.
-
Metabolomics of 3D cell co-culture reveals alterations in energy metabolism at the cross-talk of colorectal cancer-adipocytes.Front Med (Lausanne). 2024 Oct 3;11:1436866. doi: 10.3389/fmed.2024.1436866. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39421865 Free PMC article.
-
Phospholipid supplementation inhibits male and female odor discrimination in mice.Front Behav Neurosci. 2024 Jul 25;18:1397284. doi: 10.3389/fnbeh.2024.1397284. eCollection 2024. Front Behav Neurosci. 2024. PMID: 39132447 Free PMC article.
-
Proteomic, Metabolomic, and Fatty Acid Profiling of Small Extracellular Vesicles from Glioblastoma Stem-Like Cells and Their Role in Tumor Heterogeneity.ACS Nano. 2024 Jan 23;18(3):2500-2519. doi: 10.1021/acsnano.3c11427. Epub 2024 Jan 11. ACS Nano. 2024. PMID: 38207106 Free PMC article.
References
-
- Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 2009;21:209–218. - PubMed
-
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. - PubMed
-
- Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
