Acetylcholinesterase inhibitors: pharmacology and toxicology
- PMID: 24179466
- PMCID: PMC3648782
- DOI: 10.2174/1570159X11311030006
Acetylcholinesterase inhibitors: pharmacology and toxicology
Abstract
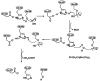
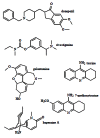
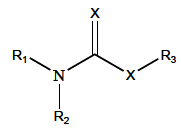
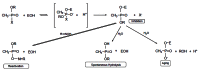
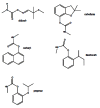
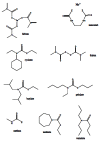
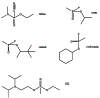
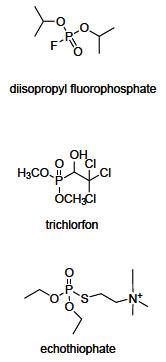
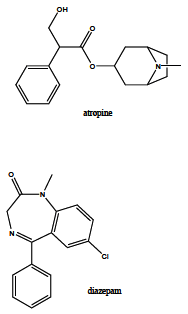
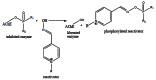
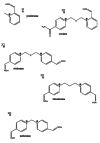
Acetylcholinesterase is involved in the termination of impulse transmission by rapid hydrolysis of the neurotransmitter acetylcholine in numerous cholinergic pathways in the central and peripheral nervous systems. The enzyme inactivation, induced by various inhibitors, leads to acetylcholine accumulation, hyperstimulation of nicotinic and muscarinic receptors, and disrupted neurotransmission. Hence, acetylcholinesterase inhibitors, interacting with the enzyme as their primary _target, are applied as relevant drugs and toxins. This review presents an overview of toxicology and pharmacology of reversible and irreversible acetylcholinesterase inactivating compounds. In the case of reversible inhibitors being commonly applied in neurodegenerative disorders treatment, special attention is paid to currently approved drugs (donepezil, rivastigmine and galantamine) in the pharmacotherapy of Alzheimer's disease, and toxic carbamates used as pesticides. Subsequently, mechanism of irreversible acetylcholinesterase inhibition induced by organophosphorus compounds (insecticides and nerve agents), and their specific and nonspecific toxic effects are described, as well as irreversible inhibitors having pharmacological implementation. In addition, the pharmacological treatment of intoxication caused by organophosphates is presented, with emphasis on oxime reactivators of the inhibited enzyme activity administering as causal drugs after the poisoning. Besides, organophosphorus and carbamate insecticides can be detoxified in mammals through enzymatic hydrolysis before they reach _targets in the nervous system. Carboxylesterases most effectively decompose carbamates, whereas the most successful route of organophosphates detoxification is their degradation by corresponding phosphotriesterases.
Keywords: Acetylcholine; Alzheimer’s disease drugs; acetylcholinesterase; carbamates; detoxification; irreversible inhibitors; organophosphates; reversible inhibitors..
Figures
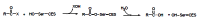
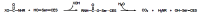
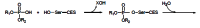
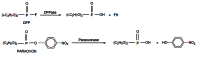
Similar articles
-
Modulators of Acetylcholinesterase Activity: From Alzheimer's Disease to Anti-Cancer Drugs.Curr Med Chem. 2017;24(30):3283-3309. doi: 10.2174/0929867324666170705123509. Curr Med Chem. 2017. PMID: 28685687 Review.
-
Cholinesterase inhibitors used in the treatment of Alzheimer's disease: the relationship between pharmacological effects and clinical efficacy.Drugs Aging. 2004;21(7):453-78. doi: 10.2165/00002512-200421070-00004. Drugs Aging. 2004. PMID: 15132713 Review.
-
The summary on non-reactivation cholinergic properties of oxime reactivators: the interaction with muscarinic and nicotinic receptors.Arch Toxicol. 2013 Apr;87(4):711-9. doi: 10.1007/s00204-012-0977-1. Epub 2012 Nov 21. Arch Toxicol. 2013. PMID: 23179755 Review.
-
Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer's disease.Clin Pharmacokinet. 2013 Apr;52(4):225-41. doi: 10.1007/s40262-013-0038-9. Clin Pharmacokinet. 2013. PMID: 23408070 Review.
-
Acetylcholinesterase Enzyme Inhibitor Molecules with Therapeutic Potential for Alzheimer's Disease.CNS Neurol Disord Drug _targets. 2022;21(5):427-449. doi: 10.2174/1871527320666210928160159. CNS Neurol Disord Drug _targets. 2022. PMID: 34602041 Review.
Cited by
-
Plant extracts as emerging modulators of neuroinflammation and immune receptors in Alzheimer's pathogenesis.Heliyon. 2024 Aug 10;10(16):e35943. doi: 10.1016/j.heliyon.2024.e35943. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39229544 Free PMC article.
-
Study of the probable genotoxic effects of Zolone (Phosalone) exposure in mice bone marrow derived cells.Genes Environ. 2021 May 13;43(1):18. doi: 10.1186/s41021-021-00191-5. Genes Environ. 2021. PMID: 33985589 Free PMC article.
-
Entomopathogenic fungi based microbial insecticides and their physiological and biochemical effects on Spodoptera frugiperda (J.E. Smith).Front Cell Infect Microbiol. 2023 Dec 11;13:1254475. doi: 10.3389/fcimb.2023.1254475. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 38149005 Free PMC article.
-
An Overview of Antennal Esterases in Lepidoptera.Front Physiol. 2021 Mar 31;12:643281. doi: 10.3389/fphys.2021.643281. eCollection 2021. Front Physiol. 2021. PMID: 33868009 Free PMC article. Review.
-
A Scientific Review of Pesticides: Classification, Toxicity, Health Effects, Sustainability, and Environmental Impact.Cureus. 2024 Aug 27;16(8):e67945. doi: 10.7759/cureus.67945. eCollection 2024 Aug. Cureus. 2024. PMID: 39328626 Free PMC article. Review.
References
-
- Massoulie J, Pezzementi L, Bon S, Krejci E, Vallette FM. Molecular and cellular biology of cholinesterases. Prog. Neurobiol. 1993;41(1 ):31–91. - PubMed
-
- Koelle GB. The histochemical localization of cholinesterases in the central nervous system of the rat. J. Comp. Anat. 1954;100(1 ):211–235. - PubMed
-
- Wang R, Tang XC. Neuroprotective Effects of Huperzine A. Neurosignals. 2005;14:71–82. - PubMed
-
- Huang YJ, Huang Y, Baldassarre H, Wang B, Lazaris A, Leduc M, Bilodeau AS, Bellemare A, Cote M, Herskovits P, Touati M, Turcotte C, Valeanu L, Lemee N, Wilgus H, Begin I, Bhatia B, Rao K, Neveu N, Brochu E, Pierson J, Hockley DK, Cerasoli DM, Lenz DE, Karatzas CN, Langermann S. Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc. Natl. Acad. Sci. U.S.A. 2007;104(34 ):13603–13608. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
