Mitochondrial DNA induces inflammation and increases TLR9/NF-κB expression in lung tissue
- PMID: 24535292
- PMCID: PMC3976143
- DOI: 10.3892/ijmm.2014.1650
Mitochondrial DNA induces inflammation and increases TLR9/NF-κB expression in lung tissue
Retraction in
-
[Retracted] Mitochondrial DNA induces inflammation and increases TLR9/NF‑κB expression in lung tissue.Int J Mol Med. 2023 Mar;51(3):28. doi: 10.3892/ijmm.2023.5231. Epub 2023 Feb 17. Int J Mol Med. 2023. PMID: 36799160 Free PMC article.
Abstract
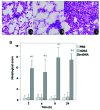
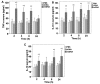
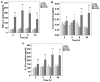
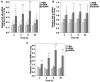
Mitochondrial DNA (mtDNA) contains unmethylated CpG motifs that exhibit immune stimulatory capacities. The aim of this study was to investigate whether mtDNA activates the Toll-like receptor 9 (TLR9)/nuclear factor-κB (NF-κB) pathway, thereby contributing to post-traumatic systemic inflammatory response syndrome (SIRS) and lung injury in rats. The effects of mtDNA on macrophage culture were examined in order to elucidate the putative cellular mechanisms. Rats and macrophage cultures were treated with phosphate-buffered saline, nuclear DNA, or mtDNA for 2, 4, 8 and 24 h. Histological analysis of lung tissue was undertaken following hematoxylin and eosin staining, and cytokine levels were assessed by ELISA. NF-κB and IκB-α phosphorylation levels, as well as TLR9 protein expression were determined by western blot analysis; NF-κB, IκB-α and TLR9 mRNA levels were analyzed by RT-PCR. A greater degree of inflammation and lung injury was observed in response to mtDNA. In addition, mtDNA increased serum tumor necrosis factor-α, interleukin (IL)-6 and IL-10 levels in vivo and increased their secretion by cultured macrophages (p<0.05). In lung tissue, mtDNA increased NF-κB, IκB-α and TLR9 mRNA levels (p<0.05); it also increased phosphorylated NF-κB p65 and TLR9 protein levels in the macrophage cultures. Thus, mtDNA may be part of the danger-associated molecular patterns, contributing to the initiation of sterile SIRS through the activation of the TLR9/NF-κB pathway and the induction of pro-inflammatory cytokine production.
Figures

Similar articles
-
[Role and mechanism of mitochondrial DNA mediated Toll-like receptor 9-myeloid differentiation factor 88 signaling pathway activation in rats with ventilator-induced lung injury].Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018 Jan;30(1):13-17. doi: 10.3760/cma.j.issn.2095-4352.2018.01.003. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2018. PMID: 29308751 Chinese.
-
Extracellular mtDNA activates NF-κB via toll-like receptor 9 and induces cell death in cardiomyocytes.Basic Res Cardiol. 2016 Jul;111(4):42. doi: 10.1007/s00395-016-0553-6. Epub 2016 May 10. Basic Res Cardiol. 2016. PMID: 27164906
-
Mitochondrial DNA induces Foley catheter related bladder inflammation via Toll-like receptor 9 activation.Sci Rep. 2018 Apr 23;8(1):6377. doi: 10.1038/s41598-018-24818-w. Sci Rep. 2018. PMID: 29686303 Free PMC article.
-
NF-κB: A Double-Edged Sword Controlling Inflammation.Biomedicines. 2022 May 27;10(6):1250. doi: 10.3390/biomedicines10061250. Biomedicines. 2022. PMID: 35740272 Free PMC article. Review.
-
The central inflammatory regulator IκBζ: induction, regulation and physiological functions.Front Immunol. 2023 Jun 12;14:1188253. doi: 10.3389/fimmu.2023.1188253. eCollection 2023. Front Immunol. 2023. PMID: 37377955 Free PMC article. Review.
Cited by
-
Accumulation of Circulating Cell-Free CpG-Enriched Ribosomal DNA Fragments on the Background of High Endonuclease Activity of Blood Plasma in Schizophrenic Patients.Int J Genomics. 2019 Aug 5;2019:8390585. doi: 10.1155/2019/8390585. eCollection 2019. Int J Genomics. 2019. PMID: 31467866 Free PMC article.
-
Mitochondrial alarmins are tissue mediators of ventilator-induced lung injury and ARDS.PLoS One. 2019 Nov 22;14(11):e0225468. doi: 10.1371/journal.pone.0225468. eCollection 2019. PLoS One. 2019. PMID: 31756204 Free PMC article.
-
Mitochondrial DNA and TLR9 drive muscle inflammation upon Opa1 deficiency.EMBO J. 2018 May 15;37(10):e96553. doi: 10.15252/embj.201796553. Epub 2018 Apr 9. EMBO J. 2018. PMID: 29632021 Free PMC article.
-
Toll-Like Receptor 9-Mediated Neuronal Innate Immune Reaction Is Associated with Initiating a Pro-Regenerative State in Neurons of the Dorsal Root Ganglia Non-Associated with Sciatic Nerve Lesion.Int J Mol Sci. 2021 Jul 12;22(14):7446. doi: 10.3390/ijms22147446. Int J Mol Sci. 2021. PMID: 34299065 Free PMC article.
-
DNA damage, metabolism and aging in pro-inflammatory T cells: Rheumatoid arthritis as a model system.Exp Gerontol. 2018 May;105:118-127. doi: 10.1016/j.exger.2017.10.027. Epub 2017 Nov 8. Exp Gerontol. 2018. PMID: 29101015 Free PMC article. Review.
References
-
- Tsukamoto T, Chanthaphavong RS, Pape HC. Current theories on the pathophysiology of multiple organ failure after trauma. Injury. 2010;41:21–26. - PubMed
-
- Kitajima I, Niimi H. Establishment of the rapid, hypersensitive testing systems for sepsis/SIRS. Rinsho Byori. 2012;60:46–51. (In Japanese) - PubMed
-
- Pittet D, Rangel-Frausto S, Li N, et al. Systemic inflammatory response syndrome, sepsis, severe sepsis and septic shock: incidence, morbidities and outcomes in surgical ICU patients. Intensive Care Med. 1995;21:302–309. - PubMed
-
- Tarlowe MH, Kannan KB, Itagaki K, Adams JM, Livingston DH, Hauser CJ. Inflammatory chemoreceptor cross-talk suppresses leukotriene B4 receptor 1-mediated neutrophil calcium mobilization and chemotaxis after trauma. J Immunol. 2003;171:2066–2073. - PubMed
-
- Bone LB, Giannoudis P. Femoral shaft fracture fixation and chest injury after polytrauma. J Bone Joint Surg Am. 2011;93:311–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

