Hydrolysis of DFP and the nerve agent (S)-sarin by DFPase proceeds along two different reaction pathways: implications for engineering bioscavengers
- PMID: 24720808
- PMCID: PMC4010294
- DOI: 10.1021/jp410422c
Hydrolysis of DFP and the nerve agent (S)-sarin by DFPase proceeds along two different reaction pathways: implications for engineering bioscavengers
Abstract
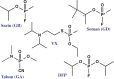
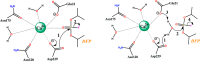
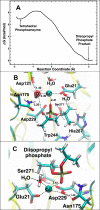
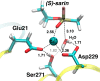
Organophosphorus (OP) nerve agents such as (S)-sarin are among the most highly toxic compounds that have been synthesized. Engineering enzymes that catalyze the hydrolysis of nerve agents ("bioscavengers") is an emerging prophylactic approach to diminish their toxic effects. Although its native function is not known, diisopropyl fluorophosphatase (DFPase) from Loligo vulgaris catalyzes the hydrolysis of OP compounds. Here, we investigate the mechanisms of diisopropylfluorophosphate (DFP) and (S)-sarin hydrolysis by DFPase with quantum mechanical/molecular mechanical umbrella sampling simulations. We find that the mechanism for hydrolysis of DFP involves nucleophilic attack by Asp229 on phosphorus to form a pentavalent intermediate. P-F bond dissociation then yields a phosphoacyl enzyme intermediate in the rate-limiting step. The simulations suggest that a water molecule, coordinated to the catalytic Ca(2+), donates a proton to Asp121 and then attacks the tetrahedral phosphoacyl intermediate to liberate the diisopropylphosphate product. In contrast, the calculated free energy barrier for hydrolysis of (S)-sarin by the same mechanism is highly unfavorable, primarily because of the instability of the pentavalent phosphoenzyme species. Instead, simulations suggest that hydrolysis of (S)-sarin proceeds by a mechanism in which Asp229 could activate an intervening water molecule for nucleophilic attack on the substrate. These findings may lead to improved strategies for engineering DFPase and related six-bladed β-propeller folds for more efficient degradation of OP compounds.
Figures



Similar articles
-
Monitoring the hydrolysis of toxic organophosphonate nerve agents in aqueous buffer and in bicontinuous microemulsions by use of diisopropyl fluorophosphatase (DFPase) with (1)H- (31)P HSQC NMR spectroscopy.Anal Bioanal Chem. 2010 Feb;396(3):1213-21. doi: 10.1007/s00216-009-3299-2. Epub 2009 Nov 27. Anal Bioanal Chem. 2010. PMID: 19943158
-
Reversed enantioselectivity of diisopropyl fluorophosphatase against organophosphorus nerve agents by rational design.J Am Chem Soc. 2009 Dec 2;131(47):17226-32. doi: 10.1021/ja905444g. J Am Chem Soc. 2009. PMID: 19894712
-
Neutron structure and mechanistic studies of diisopropyl fluorophosphatase (DFPase).Acta Crystallogr D Biol Crystallogr. 2010 Nov;66(Pt 11):1131-8. doi: 10.1107/S0907444910034013. Epub 2010 Oct 20. Acta Crystallogr D Biol Crystallogr. 2010. PMID: 21041927 Free PMC article.
-
The evolution of phosphotriesterase for decontamination and detoxification of organophosphorus chemical warfare agents.Chem Biol Interact. 2019 Aug 1;308:80-88. doi: 10.1016/j.cbi.2019.05.023. Epub 2019 May 15. Chem Biol Interact. 2019. PMID: 31100274 Free PMC article. Review.
-
Detoxification of organophosphate nerve agents by bacterial phosphotriesterase.Toxicol Appl Pharmacol. 2005 Sep 1;207(2 Suppl):459-70. doi: 10.1016/j.taap.2005.02.025. Toxicol Appl Pharmacol. 2005. PMID: 15982683 Review.
Cited by
-
Probing the Suitability of Different Ca2+ Parameters for Long Simulations of Diisopropyl Fluorophosphatase.Molecules. 2021 Sep 26;26(19):5839. doi: 10.3390/molecules26195839. Molecules. 2021. PMID: 34641383 Free PMC article.
-
Theoretical Studies Applied to the Evaluation of the DFPase Bioremediation Potential against Chemical Warfare Agents Intoxication.Int J Mol Sci. 2018 Apr 23;19(4):1257. doi: 10.3390/ijms19041257. Int J Mol Sci. 2018. PMID: 29690585 Free PMC article.
-
Aminoalcohol-Induced Activation of Organophosphorus Hydrolase (OPH) towards Diisopropylfluorophosphate (DFP).PLoS One. 2017 Jan 13;12(1):e0169937. doi: 10.1371/journal.pone.0169937. eCollection 2017. PLoS One. 2017. PMID: 28085964 Free PMC article.
-
Theoretical Studies on Catalysis Mechanisms of Serum Paraoxonase 1 and Phosphotriesterase Diisopropyl Fluorophosphatase Suggest the Alteration of Substrate Preference from Paraoxonase to DFP.Molecules. 2018 Jul 7;23(7):1660. doi: 10.3390/molecules23071660. Molecules. 2018. PMID: 29986514 Free PMC article.
-
Similar Active Sites and Mechanisms Do Not Lead to Cross-Promiscuity in Organophosphate Hydrolysis: Implications for Biotherapeutic Engineering.J Am Chem Soc. 2017 Dec 6;139(48):17533-17546. doi: 10.1021/jacs.7b09384. Epub 2017 Nov 21. J Am Chem Soc. 2017. PMID: 29113434 Free PMC article.
References
-
- Worek F.; Thiermann H.; Szinicz L.; Eyer P. Kinetic analysis of interactions between human acetylcholinesterase, structurally different organophosphorus compounds and oximes. Biochem. Pharmacol. 2004, 68, 2237–2248. - PubMed
-
- Hörnberg A.; Artursson E.; Wärme R.; Pang Y.-P.; Ekström F. Crystal structures of oxime-bound fenamiphos-acetylcholinesterases: Reactivation involving flipping of the His447 ring to form a reactive Glu334–His447–oxime triad. Biochem. Pharmacol. 2010, 79, 507–515. - PubMed
-
- Mercey G.; Verdelet T.; Saint-Andre G.; Gillon E.; Wagner A.; Baati R.; Jean L.; Nachon F.; Renard P. Y. First efficient uncharged reactivators for the dephosphylation of poisoned human acetylcholinesterase. Chem. Commun. 2011, 47, 5295–5297. - PubMed
-
- Kovach I. M. Stereochemistry and secondary reactions in the irreversible inhibition of serine hydrolases by organophosphorus compounds. J. Phys. Org. Chem. 2004, 17, 602–614.
-
- Michel H. O.; Hackley B. E.; Berkowitz L.; List G.; Hackley E. B.; Gillilan W.; Pankau M. Ageing and dealkylation of soman (pinacolylmethylphosphono-fluoridate)-inactivated eel cholinesterase. Arch. Biochem. Biophys. 1967, 121, 29–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

