The master cell cycle regulator APC-Cdc20 regulates ciliary length and disassembly of the primary cilium
- PMID: 25139956
- PMCID: PMC4135350
- DOI: 10.7554/eLife.03083
The master cell cycle regulator APC-Cdc20 regulates ciliary length and disassembly of the primary cilium
Abstract
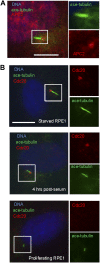
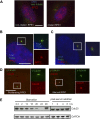
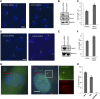
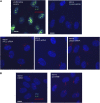
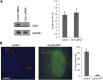
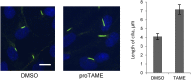
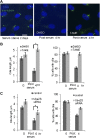
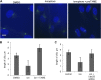
The primary cilium has an important role in signaling; defects in structure are associated with a variety of human diseases. Much of the most basic biology of this organelle is poorly understood, even basic mechanisms, such as control of growth and resorption. We show that the activity of the anaphase-promoting complex (APC), an E3 that regulates the onset of anaphase, destabilizes axonemal microtubules in the primary cilium. Furthermore, the metaphase APC co-activator, Cdc20, is specifically recruited to the basal body of primary cilia. Inhibition of APC-Cdc20 activity increases the ciliary length, while overexpression of Cdc20 suppresses cilium formation. APC-Cdc20 activity is required for the timely resorption of the cilium after serum stimulation. In addition, APC regulates the stability of axonemal microtubules through _targeting Nek1, the ciliary kinase, for proteolysis. These data demonstrate a novel function of APC beyond cell cycle control and implicate critical role of ubiquitin-mediated proteolysis in ciliary disassembly.
Keywords: cell cycle; primary cilum; retinal cell culture; ubiquitin.
Copyright © 2014, Wang et al.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures




Similar articles
-
Degradation of the human mitotic checkpoint kinase Mps1 is cell cycle-regulated by APC-cCdc20 and APC-cCdh1 ubiquitin ligases.J Biol Chem. 2010 Oct 22;285(43):32988-32998. doi: 10.1074/jbc.M110.140905. Epub 2010 Aug 20. J Biol Chem. 2010. PMID: 20729194 Free PMC article.
-
APC15 mediates CDC20 autoubiquitylation by APC/C(MCC) and disassembly of the mitotic checkpoint complex.Nat Struct Mol Biol. 2012 Nov;19(11):1116-23. doi: 10.1038/nsmb.2412. Epub 2012 Sep 24. Nat Struct Mol Biol. 2012. PMID: 23007861 Free PMC article.
-
APC/C(Cdc20) _targets E2F1 for degradation in prometaphase.Cell Cycle. 2010 Oct 1;9(19):3956-64. doi: 10.4161/cc.9.19.13162. Epub 2010 Oct 26. Cell Cycle. 2010. PMID: 20948288 Free PMC article.
-
_targeting Cdc20 as a novel cancer therapeutic strategy.Pharmacol Ther. 2015 Jul;151:141-51. doi: 10.1016/j.pharmthera.2015.04.002. Epub 2015 Apr 4. Pharmacol Ther. 2015. PMID: 25850036 Free PMC article. Review.
-
_targeting Cdc20 for cancer therapy.Biochim Biophys Acta Rev Cancer. 2022 Nov;1877(6):188824. doi: 10.1016/j.bbcan.2022.188824. Epub 2022 Oct 12. Biochim Biophys Acta Rev Cancer. 2022. PMID: 36243246 Review.
Cited by
-
TTBK2: a tau protein kinase beyond tau phosphorylation.Biomed Res Int. 2015;2015:575170. doi: 10.1155/2015/575170. Epub 2015 Apr 9. Biomed Res Int. 2015. PMID: 25950000 Free PMC article. Review.
-
A Proximity Mapping Journey into the Biology of the Mammalian Centrosome/Cilium Complex.Cells. 2020 Jun 3;9(6):1390. doi: 10.3390/cells9061390. Cells. 2020. PMID: 32503249 Free PMC article. Review.
-
Counterregulation of cAMP-directed kinase activities controls ciliogenesis.Nat Commun. 2018 Mar 26;9(1):1224. doi: 10.1038/s41467-018-03643-9. Nat Commun. 2018. PMID: 29581457 Free PMC article.
-
Mechanism of ciliary disassembly.Cell Mol Life Sci. 2016 May;73(9):1787-802. doi: 10.1007/s00018-016-2148-7. Epub 2016 Feb 11. Cell Mol Life Sci. 2016. PMID: 26869233 Free PMC article. Review.
-
Subacute Pulmonary Toxicity of Glutaraldehyde Aerosols in a Human In Vitro Airway Tissue Model.Int J Mol Sci. 2022 Oct 11;23(20):12118. doi: 10.3390/ijms232012118. Int J Mol Sci. 2022. PMID: 36292975 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

