Mitochondrial dynamics and inheritance during cell division, development and disease
- PMID: 25237825
- PMCID: PMC4250044
- DOI: 10.1038/nrm3877
Mitochondrial dynamics and inheritance during cell division, development and disease
Abstract
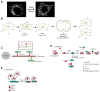
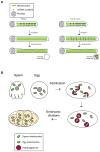
During cell division, it is critical to properly partition functional sets of organelles to each daughter cell. The partitioning of mitochondria shares some common features with that of other organelles, particularly in the use of interactions with cytoskeletal elements to facilitate delivery to the daughter cells. However, mitochondria have unique features - including their own genome and a maternal mode of germline transmission - that place additional demands on this process. Consequently, mechanisms have evolved to regulate mitochondrial segregation during cell division, oogenesis, fertilization and tissue development, as well as to ensure the integrity of these organelles and their DNA, including fusion-fission dynamics, organelle transport, mitophagy and genetic selection of functional genomes. Defects in these processes can lead to cell and tissue pathologies.
Figures

Similar articles
-
Autophagosomal Sperm Organelle Clearance and mtDNA Inheritance in C. elegans.Adv Anat Embryol Cell Biol. 2019;231:1-23. doi: 10.1007/102_2018_1. Adv Anat Embryol Cell Biol. 2019. PMID: 30467692 Review.
-
Biased placement of Mitochondria fission facilitates asymmetric inheritance of protein aggregates during yeast cell division.PLoS Comput Biol. 2023 Nov 27;19(11):e1011588. doi: 10.1371/journal.pcbi.1011588. eCollection 2023 Nov. PLoS Comput Biol. 2023. PMID: 38011208 Free PMC article.
-
Incompatibility between mitochondrial and nuclear genomes during oogenesis results in ovarian failure and embryonic lethality.Development. 2017 Jul 1;144(13):2490-2503. doi: 10.1242/dev.151951. Epub 2017 Jun 2. Development. 2017. PMID: 28576772 Free PMC article.
-
Fusion, fission, and transport control asymmetric inheritance of mitochondria and protein aggregates.J Cell Biol. 2017 Aug 7;216(8):2481-2498. doi: 10.1083/jcb.201611197. Epub 2017 Jun 14. J Cell Biol. 2017. PMID: 28615194 Free PMC article.
-
Yeast mitochondrial dynamics: fusion, division, segregation, and shape.Microsc Res Tech. 2000 Dec 15;51(6):573-83. doi: 10.1002/1097-0029(20001215)51:6<573::AID-JEMT7>3.0.CO;2-2. Microsc Res Tech. 2000. PMID: 11169859 Review.
Cited by
-
Using mitochondrial activity to select for potent human hematopoietic stem cells.Blood Adv. 2021 Mar 23;5(6):1605-1616. doi: 10.1182/bloodadvances.2020003658. Blood Adv. 2021. PMID: 33710339 Free PMC article.
-
The transcriptional coactivator CBP/p300 is an evolutionarily conserved node that promotes longevity in response to mitochondrial stress.Nat Aging. 2021 Feb;1(2):165-178. doi: 10.1038/s43587-020-00025-z. Epub 2021 Feb 8. Nat Aging. 2021. PMID: 33718883 Free PMC article.
-
Mitochondrial Function, Biology, and Role in Disease: A Scientific Statement From the American Heart Association.Circ Res. 2016 Jun 10;118(12):1960-91. doi: 10.1161/RES.0000000000000104. Epub 2016 Apr 28. Circ Res. 2016. PMID: 27126807 Free PMC article. Review.
-
CYP27A1-dependent anti-melanoma activity of limonoid natural products _targets mitochondrial metabolism.Cell Chem Biol. 2021 Oct 21;28(10):1407-1419.e6. doi: 10.1016/j.chembiol.2021.03.004. Epub 2021 Mar 31. Cell Chem Biol. 2021. PMID: 33794192 Free PMC article.
-
Num1 anchors mitochondria to the plasma membrane via two domains with different lipid binding specificities.J Cell Biol. 2016 Jun 6;213(5):513-24. doi: 10.1083/jcb.201511021. Epub 2016 May 30. J Cell Biol. 2016. PMID: 27241910 Free PMC article.
References
-
- Scheffler IE. Mitochondria. John Wiley & Sons, Inc; Hoboken, NJ: 2009.
-
- Stehling O, Wilbrecht C, Lill R. Mitochondrial iron-sulfur protein biogenesis and human disease. Biochimie. 2014;100C:61–77. - PubMed
-
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–32. - PubMed
-
- Gray MW, Burger G, Lang BF. Mitochondrial evolution. Science. 1999;283:1476–81. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

