Hmga2 regulates self-renewal of retinal progenitors
- PMID: 25336737
- PMCID: PMC4302892
- DOI: 10.1242/dev.107326
Hmga2 regulates self-renewal of retinal progenitors
Abstract
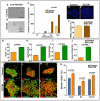
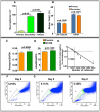
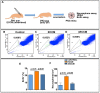
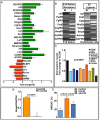
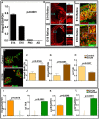
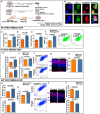
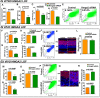
In vertebrate retina, histogenesis occurs over an extended period. To sustain the temporal generation of diverse cell types, retinal progenitor cells (RPCs) must self-renew. However, self-renewal and regulation of RPCs remain poorly understood. Here, we demonstrate that cell-extrinsic factors coordinate with the epigenetic regulator high-mobility group AT-hook 2 (Hmga2) to regulate self-renewal of late retinal progenitor cells (RPCs). We observed that a small subset of RPCs was capable of clonal propagation and retained multipotentiality of parents in the presence of endothelial cells (ECs), known self-renewal regulators in various stem cell niches. The self-renewing effects, also observed in vivo, involve multiple intercellular signaling pathways, engaging Hmga2. As progenitors exhaust during retinal development, expression of Hmga2 progressively decreases. Analyses of Hmga2-expression perturbation, in vitro and in vivo, revealed that Hmga2 functionally helps to mediate cell-extrinsic influences on late-retinal progenitor self-renewal. Our results provide a framework for integrating the diverse intercellular influences elicited by epigenetic regulators for self-renewal in a dynamic stem cell niche: the developing vertebrate retina.
Keywords: Epigenetic; Hmga2; Progenitors; Rat; Retina; Self-renewal; Stem cells.
© 2014. Published by The Company of Biologists Ltd.
Figures
Similar articles
-
Bmi1 distinguishes immature retinal progenitor/stem cells from the main progenitor cell population and is required for normal retinal development.Stem Cells. 2010 Aug;28(8):1412-23. doi: 10.1002/stem.462. Stem Cells. 2010. PMID: 20549707
-
let-7 microRNA regulates neurogliogenesis in the mammalian retina through Hmga2.Dev Biol. 2016 Feb 1;410(1):70-85. doi: 10.1016/j.ydbio.2015.12.010. Epub 2015 Dec 15. Dev Biol. 2016. PMID: 26698218
-
Isolation of retinal progenitor and stem cells from the porcine eye.Mol Vis. 2007 Jun 29;13:1045-57. Mol Vis. 2007. PMID: 17653049 Free PMC article.
-
Control of cell proliferation by neurotransmitters in the developing vertebrate retina.Brain Res. 2008 Feb 4;1192:37-60. doi: 10.1016/j.brainres.2007.04.076. Epub 2007 May 6. Brain Res. 2008. PMID: 17597590 Review.
-
Cell-intrinsic regulators of proliferation in vertebrate retinal progenitors.Semin Cell Dev Biol. 2004 Feb;15(1):63-74. doi: 10.1016/j.semcdb.2003.09.001. Semin Cell Dev Biol. 2004. PMID: 15036209 Review.
Cited by
-
Rapid and Integrative Discovery of Retina Regulatory Molecules.Cell Rep. 2018 Aug 28;24(9):2506-2519. doi: 10.1016/j.celrep.2018.07.090. Cell Rep. 2018. PMID: 30157441 Free PMC article.
-
Let-7 Represses Carcinogenesis and a Stem Cell Phenotype in the Intestine via Regulation of Hmga2.PLoS Genet. 2015 Aug 5;11(8):e1005408. doi: 10.1371/journal.pgen.1005408. eCollection 2015 Aug. PLoS Genet. 2015. PMID: 26244988 Free PMC article.
-
Identification of in vivo DNA-binding mechanisms of Pax6 and reconstruction of Pax6-dependent gene regulatory networks during forebrain and lens development.Nucleic Acids Res. 2015 Aug 18;43(14):6827-46. doi: 10.1093/nar/gkv589. Epub 2015 Jul 2. Nucleic Acids Res. 2015. PMID: 26138486 Free PMC article.
-
Abnormal retinal development in Cloche mutant zebrafish.Dev Dyn. 2015 Nov;244(11):1439-1455. doi: 10.1002/dvdy.24322. Epub 2015 Sep 2. Dev Dyn. 2015. PMID: 26283463 Free PMC article.
-
HMGA2 regulates CD44 expression to promote gastric cancer cell motility and sphere formation.Am J Cancer Res. 2017 Feb 1;7(2):260-274. eCollection 2017. Am J Cancer Res. 2017. PMID: 28337375 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

