Lysosomal physiology
- PMID: 25668017
- PMCID: PMC4524569
- DOI: 10.1146/annurev-physiol-021014-071649
Lysosomal physiology
Abstract
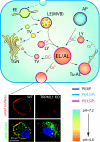
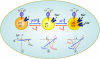
Lysosomes are acidic compartments filled with more than 60 different types of hydrolases. They mediate the degradation of extracellular particles from endocytosis and of intracellular components from autophagy. The digested products are transported out of the lysosome via specific catabolite exporters or via vesicular membrane trafficking. Lysosomes also contain more than 50 membrane proteins and are equipped with the machinery to sense nutrient availability, which determines the distribution, number, size, and activity of lysosomes to control the specificity of cargo flux and timing (the initiation and termination) of degradation. Defects in degradation, export, or trafficking result in lysosomal dysfunction and lysosomal storage diseases (LSDs). Lysosomal channels and transporters mediate ion flux across perimeter membranes to regulate lysosomal ion homeostasis, membrane potential, catabolite export, membrane trafficking, and nutrient sensing. Dysregulation of lysosomal channels underlies the pathogenesis of many LSDs and possibly that of metabolic and common neurodegenerative diseases.
Keywords: TFEB; TPC1; TPC2; TRPML1; lysosomal exocytosis; lysosomal storage disease; mTOR.
Figures

Similar articles
-
Lysosomal Ion Channels as Decoders of Cellular Signals.Trends Biochem Sci. 2019 Feb;44(2):110-124. doi: 10.1016/j.tibs.2018.10.006. Epub 2018 Nov 10. Trends Biochem Sci. 2019. PMID: 30424907 Free PMC article. Review.
-
Regulation of lysosomal ion homeostasis by channels and transporters.Sci China Life Sci. 2016 Aug;59(8):777-91. doi: 10.1007/s11427-016-5090-x. Epub 2016 Jul 19. Sci China Life Sci. 2016. PMID: 27430889 Free PMC article. Review.
-
The lysosome: from waste bag to potential therapeutic _target.J Mol Cell Biol. 2013 Aug;5(4):214-26. doi: 10.1093/jmcb/mjt022. J Mol Cell Biol. 2013. PMID: 23918283 Review.
-
TRPML1-/TFEB-Dependent Regulation of Lysosomal Exocytosis.Methods Mol Biol. 2019;1925:143-144. doi: 10.1007/978-1-4939-9018-4_12. Methods Mol Biol. 2019. PMID: 30674023
-
The role of lysosomal ion channels in lysosome dysfunction.Inhal Toxicol. 2021 Feb;33(2):41-54. doi: 10.1080/08958378.2021.1876188. Epub 2021 Feb 25. Inhal Toxicol. 2021. PMID: 33627009 Review.
Cited by
-
Neurodegeneration Upon Dysfunction of Endosomal/Lysosomal CLC Chloride Transporters.Front Cell Dev Biol. 2021 Feb 23;9:639231. doi: 10.3389/fcell.2021.639231. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33708769 Free PMC article. Review.
-
Cathepsin B modulates lysosomal biogenesis and host defense against Francisella novicida infection.J Exp Med. 2016 Sep 19;213(10):2081-97. doi: 10.1084/jem.20151938. Epub 2016 Aug 22. J Exp Med. 2016. PMID: 27551156 Free PMC article.
-
A tale of two CLCs: biophysical insights toward understanding ClC-5 and ClC-7 function in endosomes and lysosomes.J Physiol. 2015 Sep 15;593(18):4139-50. doi: 10.1113/JP270604. Epub 2015 Jun 26. J Physiol. 2015. PMID: 26036722 Free PMC article. Review.
-
Identification of the genetic central dogma in osteogenic differentiation of MSCs by osteoinductive medium from transcriptional data sets.Chronic Dis Transl Med. 2022 May 31;8(3):218-228. doi: 10.1002/cdt3.26. eCollection 2022 Sep. Chronic Dis Transl Med. 2022. PMID: 36161200 Free PMC article.
-
Lysosomal positioning diseases: beyond substrate storage.Open Biol. 2022 Oct;12(10):220155. doi: 10.1098/rsob.220155. Epub 2022 Oct 26. Open Biol. 2022. PMID: 36285443 Free PMC article. Review.
References
-
- Luzio JP, Pryor PR, Bright NA. Lysosomes: fusion and function. Nat. Rev. Mol. Cell Biol. 2007;8:622–32. - PubMed
-
- Kolter T, Sandhoff K. Principles of lysosomal membrane digestion: stimulation of sphingolipid degradation by sphingolipid activator proteins and anionic lysosomal lipids. Annu. Rev. Cell Dev. Biol. 2005;21:81–103. - PubMed
-
- Ruivo R, Anne C, Sagne C, Gasnier B. Molecular and cellular basis of lysosomal transmembrane protein dysfunction. Biochim. Biophys. Acta. 2009;1793:636–49. - PubMed
-
- Saftig P, Klumperman J. Lysosome biogenesis and lysosomal membrane proteins: Trafficking meets function. Nat. Rev. Mol. Cell Biol. 2009;10:623–35. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

