_targeting mitochondrial reactive oxygen species to modulate hypoxia-induced pulmonary hypertension
- PMID: 26073127
- PMCID: PMC4615392
- DOI: 10.1016/j.freeradbiomed.2015.05.042
_targeting mitochondrial reactive oxygen species to modulate hypoxia-induced pulmonary hypertension
Abstract
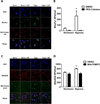
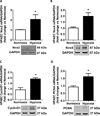
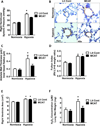
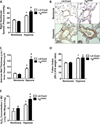
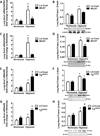
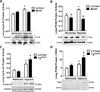
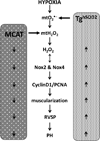
Pulmonary hypertension (PH) is characterized by increased pulmonary vascular remodeling, resistance, and pressures. Reactive oxygen species (ROS) contribute to PH-associated vascular dysfunction. NADPH oxidases (Nox) and mitochondria are major sources of superoxide (O(2)(•-)) and hydrogen peroxide (H(2)O(2)) in pulmonary vascular cells. Hypoxia, a common stimulus of PH, increases Nox expression and mitochondrial ROS (mtROS) production. The interactions between these two sources of ROS generation continue to be defined. We hypothesized that mitochondria-derived O(2)(•-) (mtO(2)(•-)) and H(2)O(2) (mtH(2)O(2)) increase Nox expression to promote PH pathogenesis and that mitochondria-_targeted antioxidants can reduce mtROS, Nox expression, and hypoxia-induced PH. Exposure of human pulmonary artery endothelial cells to hypoxia for 72 h increased mtO(2)(•-) and mtH(2)O(2). To assess the contribution of mtO(2)(•-) and mtH(2)O(2) to hypoxia-induced PH, mice that overexpress superoxide dismutase 2 (Tg(hSOD2)) or mitochondria-_targeted catalase (MCAT) were exposed to normoxia (21% O(2)) or hypoxia (10% O(2)) for three weeks. Compared with hypoxic control mice, MCAT mice developed smaller hypoxia-induced increases in RVSP, α-SMA staining, extracellular H(2)O(2) (Amplex Red), Nox2 and Nox4 (qRT-PCR and Western blot), or cyclinD1 and PCNA (Western blot). In contrast, Tg(hSOD2) mice experienced exacerbated responses to hypoxia. These studies demonstrate that hypoxia increases mtO(2)(•-) and mtH(2)O(2). _targeting mtH(2)O(2) attenuates PH pathogenesis, whereas _targeting mtO(2)(•-) exacerbates PH. These differences in PH pathogenesis were mirrored by RVSP, vessel muscularization, levels of Nox2 and Nox4, proliferation, and H(2)O(2) release. These studies suggest that _targeted reductions in mtH(2)O(2) generation may be particularly effective in preventing hypoxia-induced PH.
Keywords: Catalase; Hydrogen peroxide; Mitochondria; NADPH oxidase; Pulmonary hypertension; ROS; SOD2; Superoxide.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures
Similar articles
-
The Nox4 inhibitor GKT137831 attenuates hypoxia-induced pulmonary vascular cell proliferation.Am J Respir Cell Mol Biol. 2012 Nov;47(5):718-26. doi: 10.1165/rcmb.2011-0418OC. Epub 2012 Aug 16. Am J Respir Cell Mol Biol. 2012. PMID: 22904198 Free PMC article.
-
Nox-derived ROS are acutely activated in pressure overload pulmonary hypertension: indications for a seminal role for mitochondrial Nox4.Am J Physiol Heart Circ Physiol. 2014 Jan 15;306(2):H197-205. doi: 10.1152/ajpheart.00977.2012. Epub 2013 Nov 8. Am J Physiol Heart Circ Physiol. 2014. PMID: 24213612 Free PMC article.
-
Hypoxia inhibits expression and function of mitochondrial thioredoxin 2 to promote pulmonary hypertension.Am J Physiol Lung Cell Mol Physiol. 2017 May 1;312(5):L599-L608. doi: 10.1152/ajplung.00258.2016. Epub 2017 Jan 27. Am J Physiol Lung Cell Mol Physiol. 2017. PMID: 28130258 Free PMC article.
-
Reactive oxygen species as therapeutic _targets in pulmonary hypertension.Ther Adv Respir Dis. 2013 Jun;7(3):175-200. doi: 10.1177/1753465812472940. Epub 2013 Jan 17. Ther Adv Respir Dis. 2013. PMID: 23328248 Review.
-
Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer.Am J Physiol Heart Circ Physiol. 2008 Feb;294(2):H570-8. doi: 10.1152/ajpheart.01324.2007. Epub 2007 Dec 14. Am J Physiol Heart Circ Physiol. 2008. PMID: 18083891 Review.
Cited by
-
NADPH oxidase-2 mediates zinc deficiency-induced oxidative stress and kidney damage.Am J Physiol Cell Physiol. 2017 Jan 1;312(1):C47-C55. doi: 10.1152/ajpcell.00208.2016. Epub 2016 Nov 2. Am J Physiol Cell Physiol. 2017. PMID: 27806940 Free PMC article.
-
Mitochondrial Regulation of the Hypoxia-Inducible Factor in the Development of Pulmonary Hypertension.J Clin Med. 2022 Sep 3;11(17):5219. doi: 10.3390/jcm11175219. J Clin Med. 2022. PMID: 36079149 Free PMC article. Review.
-
NADPH oxidase: its potential role in promotion of pulmonary arterial hypertension.Naunyn Schmiedebergs Arch Pharmacol. 2017 Apr;390(4):331-338. doi: 10.1007/s00210-017-1359-2. Epub 2017 Feb 11. Naunyn Schmiedebergs Arch Pharmacol. 2017. PMID: 28190244 Review.
-
Mitochondria in lung disease.J Clin Invest. 2016 Mar 1;126(3):809-20. doi: 10.1172/JCI81113. Epub 2016 Mar 1. J Clin Invest. 2016. PMID: 26928034 Free PMC article. Review.
-
Identification of Hypoxia Induced Metabolism Associated Genes in Pulmonary Hypertension.Front Pharmacol. 2021 Nov 5;12:753727. doi: 10.3389/fphar.2021.753727. eCollection 2021. Front Pharmacol. 2021. PMID: 34803695 Free PMC article.
References
-
- Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. American journal of physiology. Lung cellular and molecular physiology. 2008;295:L881–L888. - PMC - PubMed
-
- Liu JQ, Folz RJ. Extracellular superoxide enhances 5-HT-induced murine pulmonary artery vasoconstriction. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2004;287:L111–L118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

