Flavonoids and Auxin Transport Inhibitors Rescue Symbiotic Nodulation in the Medicago truncatula Cytokinin Perception Mutant cre1
- PMID: 26253705
- PMCID: PMC4568502
- DOI: 10.1105/tpc.15.00231
Flavonoids and Auxin Transport Inhibitors Rescue Symbiotic Nodulation in the Medicago truncatula Cytokinin Perception Mutant cre1
Abstract
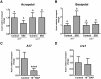
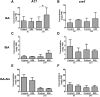
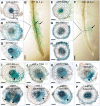
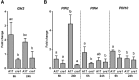
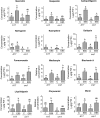
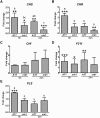
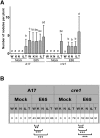
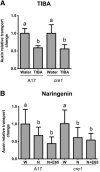
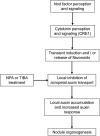
Initiation of symbiotic nodules in legumes requires cytokinin signaling, but its mechanism of action is largely unknown. Here, we tested whether the failure to initiate nodules in the Medicago truncatula cytokinin perception mutant cre1 (cytokinin response1) is due to its altered ability to regulate auxin transport, auxin accumulation, and induction of flavonoids. We found that in the cre1 mutant, symbiotic rhizobia cannot locally alter acro- and basipetal auxin transport during nodule initiation and that these mutants show reduced auxin (indole-3-acetic acid) accumulation and auxin responses compared with the wild type. Quantification of flavonoids, which can act as endogenous auxin transport inhibitors, showed a deficiency in the induction of free naringenin, isoliquiritigenin, quercetin, and hesperetin in cre1 roots compared with wild-type roots 24 h after inoculation with rhizobia. Coinoculation of roots with rhizobia and the flavonoids naringenin, isoliquiritigenin, and kaempferol, or with the synthetic auxin transport inhibitor 2,3,5,-triiodobenzoic acid, rescued nodulation efficiency in cre1 mutants and allowed auxin transport control in response to rhizobia. Our results suggest that CRE1-dependent cytokinin signaling leads to nodule initiation through the regulation of flavonoid accumulation required for local alteration of polar auxin transport and subsequent auxin accumulation in cortical cells during the early stages of nodulation.
© 2015 American Society of Plant Biologists. All rights reserved.
Figures

Similar articles
-
Rhizobial infection is associated with the development of peripheral vasculature in nodules of Medicago truncatula.Plant Physiol. 2013 May;162(1):107-15. doi: 10.1104/pp.113.215111. Epub 2013 Mar 27. Plant Physiol. 2013. PMID: 23535942 Free PMC article.
-
MtCRE1-dependent cytokinin signaling integrates bacterial and plant cues to coordinate symbiotic nodule organogenesis in Medicago truncatula.Plant J. 2011 Feb;65(4):622-33. doi: 10.1111/j.1365-313X.2010.04447.x. Epub 2011 Jan 18. Plant J. 2011. PMID: 21244535
-
Sucrose-induced auxin conjugate hydrolase restores symbiosis in a Medicago cytokinin perception mutant.Plant Physiol. 2023 Apr 3;191(4):2447-2460. doi: 10.1093/plphys/kiad045. Plant Physiol. 2023. PMID: 36722159 Free PMC article.
-
The Control of Auxin Transport in Parasitic and Symbiotic Root-Microbe Interactions.Plants (Basel). 2015 Aug 24;4(3):606-43. doi: 10.3390/plants4030606. Plants (Basel). 2015. PMID: 27135343 Free PMC article. Review.
-
Friends in Arms: Flavonoids and the Auxin/Cytokinin Balance in Terrestrialization.Plants (Basel). 2023 Jan 23;12(3):517. doi: 10.3390/plants12030517. Plants (Basel). 2023. PMID: 36771601 Free PMC article. Review.
Cited by
-
The MYB Activator WHITE PETAL1 Associates with MtTT8 and MtWD40-1 to Regulate Carotenoid-Derived Flower Pigmentation in Medicago truncatula.Plant Cell. 2019 Nov;31(11):2751-2767. doi: 10.1105/tpc.19.00480. Epub 2019 Sep 17. Plant Cell. 2019. PMID: 31530734 Free PMC article.
-
MtLAX2, a Functional Homologue of the Arabidopsis Auxin Influx Transporter AUX1, Is Required for Nodule Organogenesis.Plant Physiol. 2017 May;174(1):326-338. doi: 10.1104/pp.16.01473. Epub 2017 Mar 31. Plant Physiol. 2017. PMID: 28363992 Free PMC article.
-
A Stringent-Response-Defective Bradyrhizobium diazoefficiens Strain Does Not Activate the Type 3 Secretion System, Elicits an Early Plant Defense Response, and Circumvents NH4NO3-Induced Inhibition of Nodulation.Appl Environ Microbiol. 2021 Apr 13;87(9):e02989-20. doi: 10.1128/AEM.02989-20. Print 2021 Apr 13. Appl Environ Microbiol. 2021. PMID: 33608284 Free PMC article.
-
Evolutionary studies of the bHLH transcription factors belonging to MBW complex: their role in seed development.Ann Bot. 2023 Nov 23;132(3):383-400. doi: 10.1093/aob/mcad097. Ann Bot. 2023. PMID: 37467144 Free PMC article. Review.
-
Genomic history of the origin and domestication of common bean unveils its closest sister species.Genome Biol. 2017 Mar 29;18(1):60. doi: 10.1186/s13059-017-1190-6. Genome Biol. 2017. PMID: 28356141 Free PMC article.
References
-
- Allen E.K., Allen O.N., Newman A.S. (1953). Pseudonodulation of leguminous plants induced by 2-bromo-3,5-dichlorobenzoic acid. Am. J. Bot. 40: 429–435.
-
- Bailly A., Sovero V., Vincenzetti V., Santelia D., Bartnik D., Koenig B.W., Mancuso S., Martinoia E., Geisler M. (2008). Modulation of P-glycoproteins by auxin transport inhibitors is mediated by interaction with immunophilins. J. Biol. Chem. 283: 21817–21826. - PubMed
-
- Benková E., Michniewicz M., Sauer M., Teichmann T., Seifertová D., Jürgens G., Friml J. (2003). Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

