Arabidopsis HECATE genes function in phytohormone control during gynoecium development
- PMID: 26293302
- PMCID: PMC4631749
- DOI: 10.1242/dev.120444
Arabidopsis HECATE genes function in phytohormone control during gynoecium development
Abstract
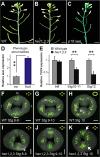
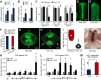
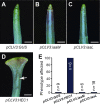
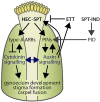
The fruit, which develops from the fertilised gynoecium formed in the innermost whorl of the flower, is the reproductive organ and one of the most complex structures of an angiosperm plant. Phytohormones play important roles during flower and fruit patterning, morphogenesis and growth, and there is emerging evidence for a cross-talk between different classes of plant hormones throughout these processes. Here, we show that the bHLH transcription factors HECATE 1 (HEC1), HEC2 and HEC3, which have previously been identified as essential components of transmitting tract formation, affect both auxin and cytokinin responses during reproductive tissue development. We find that HEC1 interacts with SPATULA (SPT) to control carpel fusion and that both transcription factors restrict sensitivity to cytokinin in the gynoecium. In addition, HEC1 is tightly integrated into the auxin-signalling network at the levels of biosynthesis, transport and transcriptional response. Based on this data, we propose that HEC1 acts as a local modulator of auxin and cytokinin responses to control gynoecium development in Arabidopsis.
Keywords: Arabidopsis; Auxin; Cytokinin; Gynoecium development; HECATE; Phytohormones; SPATULA.
© 2015. Published by The Company of Biologists Ltd.
Figures



Similar articles
-
The bHLH transcription factor SPATULA enables cytokinin signaling, and both activate auxin biosynthesis and transport genes at the medial domain of the gynoecium.PLoS Genet. 2017 Apr 7;13(4):e1006726. doi: 10.1371/journal.pgen.1006726. eCollection 2017 Apr. PLoS Genet. 2017. PMID: 28388635 Free PMC article.
-
Hormonal control of the development of the gynoecium.Curr Opin Plant Biol. 2016 Feb;29:104-14. doi: 10.1016/j.pbi.2015.12.006. Epub 2016 Jan 19. Curr Opin Plant Biol. 2016. PMID: 26799132 Review.
-
The HECATE genes regulate female reproductive tract development in Arabidopsis thaliana.Development. 2007 Oct;134(20):3593-601. doi: 10.1242/dev.011510. Epub 2007 Sep 12. Development. 2007. PMID: 17855426
-
The role of cytokinin during Arabidopsis gynoecia and fruit morphogenesis and patterning.Plant J. 2012 Oct;72(2):222-34. doi: 10.1111/j.1365-313X.2012.05062.x. Epub 2012 Aug 7. Plant J. 2012. PMID: 22640521
-
Auxin and the Arabidopsis thaliana gynoecium.J Exp Bot. 2013 Jun;64(9):2619-27. doi: 10.1093/jxb/ert099. Epub 2013 Apr 12. J Exp Bot. 2013. PMID: 23585670 Review.
Cited by
-
Transcriptomic and Physiological Analyses for the Role of Hormones and Sugar in Axillary Bud Development of Wild Strawberry Stolon.Plants (Basel). 2024 Aug 13;13(16):2241. doi: 10.3390/plants13162241. Plants (Basel). 2024. PMID: 39204677 Free PMC article.
-
The role of D3-type cyclins is related to cytokinin and the bHLH transcription factor SPATULA in Arabidopsis gynoecium development.Planta. 2024 Jul 9;260(2):48. doi: 10.1007/s00425-024-04481-4. Planta. 2024. PMID: 38980389 Free PMC article.
-
The Ins and Outs of Homeodomain-Leucine Zipper/Hormone Networks in the Regulation of Plant Development.Int J Mol Sci. 2024 May 23;25(11):5657. doi: 10.3390/ijms25115657. Int J Mol Sci. 2024. PMID: 38891845 Free PMC article. Review.
-
Arabidopsis transcription factor TCP4 controls the identity of the apical gynoecium.Plant Cell. 2024 Jul 2;36(7):2668-2688. doi: 10.1093/plcell/koae107. Plant Cell. 2024. PMID: 38581433
-
Multi-locus genome-wide association studies reveal the dynamic genetic architecture of flowering time in chrysanthemum.Plant Cell Rep. 2024 Mar 6;43(4):84. doi: 10.1007/s00299-024-03172-4. Plant Cell Rep. 2024. PMID: 38448703
References
-
- Alvarez J. and Smyth D. R. (1999). CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development 126, 2377-2386. - PubMed
-
- Alvarez J. and Smyth D. R. (2002). CRABS CLAW and SPATULA genes regulate growth and pattern formation during gynoecium development in Arabidopsis thaliana. Int. J. Plant Sci. 163, 17-41. 10.1086/324178 - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

