The relative importance of kinetic mechanisms and variable enzyme abundances for the regulation of hepatic glucose metabolism--insights from mathematical modeling
- PMID: 26935066
- PMCID: PMC4774192
- DOI: 10.1186/s12915-016-0237-6
The relative importance of kinetic mechanisms and variable enzyme abundances for the regulation of hepatic glucose metabolism--insights from mathematical modeling
Abstract
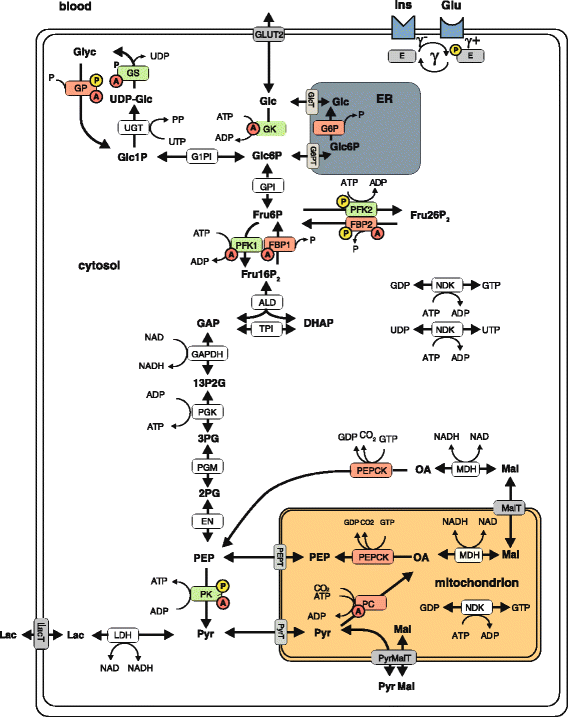
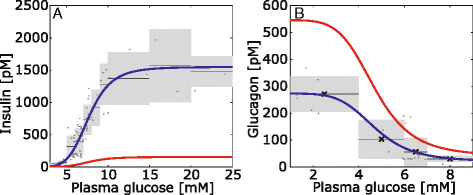
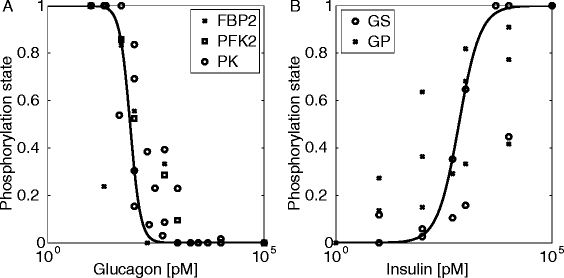
Background: Adaptation of the cellular metabolism to varying external conditions is brought about by regulated changes in the activity of enzymes and transporters. Hormone-dependent reversible enzyme phosphorylation and concentration changes of reactants and allosteric effectors are the major types of rapid kinetic enzyme regulation, whereas on longer time scales changes in protein abundance may also become operative. Here, we used a comprehensive mathematical model of the hepatic glucose metabolism of rat hepatocytes to decipher the relative importance of different regulatory modes and their mutual interdependencies in the hepatic control of plasma glucose homeostasis.
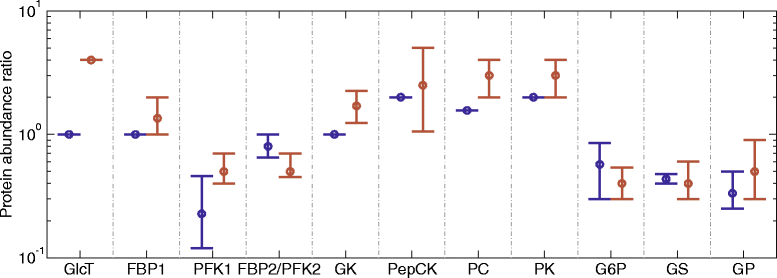
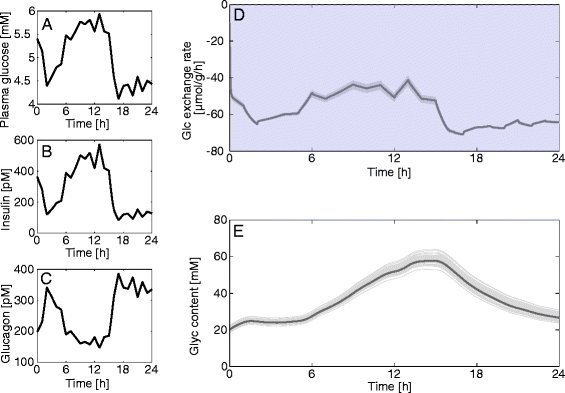
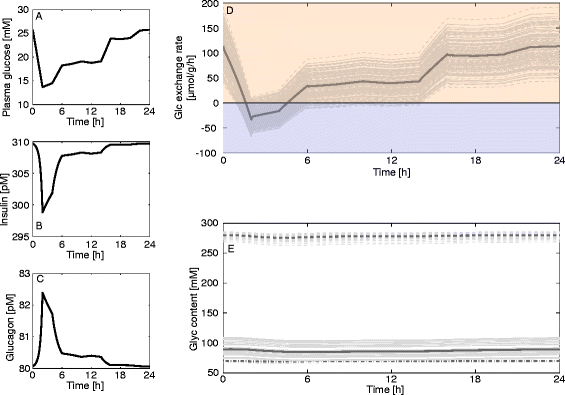
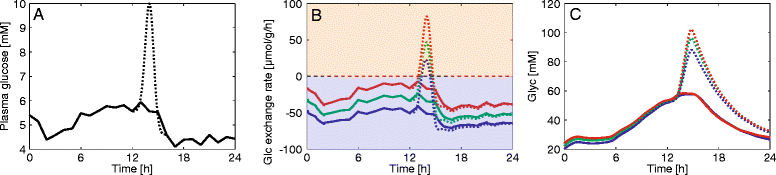
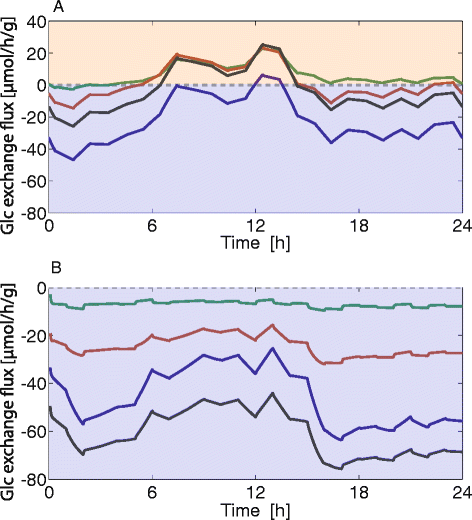
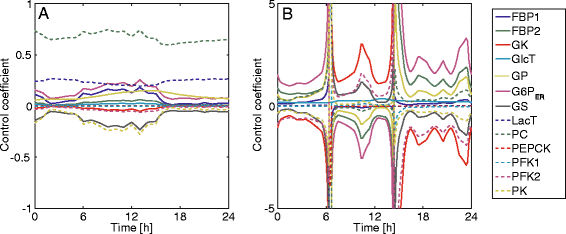
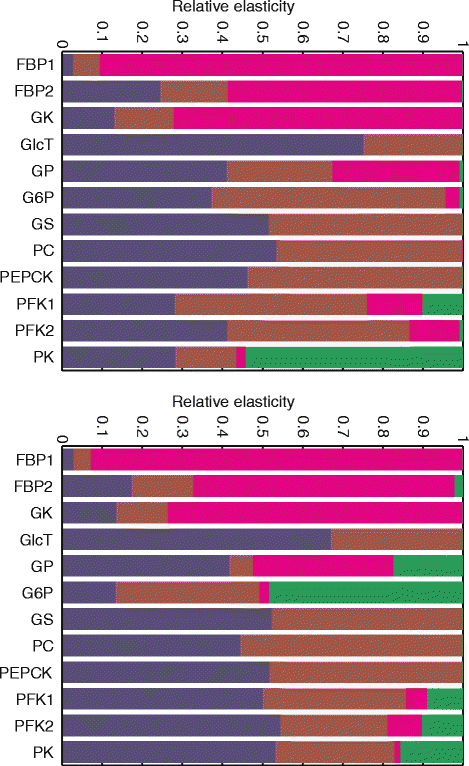
Results: Model simulations reveal significant differences in the capability of liver metabolism to counteract variations of plasma glucose in different physiological settings (starvation, ad libitum nutrient supply, diabetes). Changes in enzyme abundances adjust the metabolic output to the anticipated physiological demand but may turn into a regulatory disadvantage if sudden unexpected changes of the external conditions occur. Allosteric and hormonal control of enzyme activities allow the liver to assume a broad range of metabolic states and may even fully reverse flux changes resulting from changes of enzyme abundances alone. Metabolic control analysis reveals that control of the hepatic glucose metabolism is mainly exerted by enzymes alone, which are differently controlled by alterations in enzyme abundance, reversible phosphorylation, and allosteric effects.
Conclusion: In hepatic glucose metabolism, regulation of enzyme activities by changes of reactants, allosteric effects, and reversible phosphorylation is equally important as changes in protein abundance of key regulatory enzymes.
Figures
Similar articles
-
Kinetic modeling of human hepatic glucose metabolism in type 2 diabetes mellitus predicts higher risk of hypoglycemic events in rigorous insulin therapy.J Biol Chem. 2012 Oct 26;287(44):36978-89. doi: 10.1074/jbc.M112.382069. Epub 2012 Sep 12. J Biol Chem. 2012. PMID: 22977253 Free PMC article.
-
Quantifying the contribution of the liver to glucose homeostasis: a detailed kinetic model of human hepatic glucose metabolism.PLoS Comput Biol. 2012;8(6):e1002577. doi: 10.1371/journal.pcbi.1002577. Epub 2012 Jun 21. PLoS Comput Biol. 2012. PMID: 22761565 Free PMC article.
-
A multiscale modelling approach to assess the impact of metabolic zonation and microperfusion on the hepatic carbohydrate metabolism.PLoS Comput Biol. 2018 Feb 15;14(2):e1006005. doi: 10.1371/journal.pcbi.1006005. eCollection 2018 Feb. PLoS Comput Biol. 2018. PMID: 29447152 Free PMC article.
-
Sweet changes: glucose homeostasis can be altered by manipulating genes controlling hepatic glucose metabolism.Mol Endocrinol. 2004 May;18(5):1051-63. doi: 10.1210/me.2003-0357. Epub 2003 Dec 23. Mol Endocrinol. 2004. PMID: 14694084 Review.
-
Effects of glucagon-like peptide 1 on the hepatic glucose metabolism.Horm Metab Res. 2004 Nov-Dec;36(11-12):837-41. doi: 10.1055/s-2004-826172. Horm Metab Res. 2004. PMID: 15655716 Review.
Cited by
-
Pregnancy alters fatty acid metabolism, glucose regulation, and detoxification of the liver in synchrony with biomechanical property changes.Heliyon. 2024 Oct 22;10(20):e39674. doi: 10.1016/j.heliyon.2024.e39674. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39506943 Free PMC article.
-
Metabolic Adaptation in Epilepsy: From Acute Response to Chronic Impairment.Int J Mol Sci. 2024 Sep 6;25(17):9640. doi: 10.3390/ijms25179640. Int J Mol Sci. 2024. PMID: 39273587 Free PMC article.
-
Neurofibromin 1 controls metabolic balance and Notch-dependent quiescence of murine juvenile myogenic progenitors.Nat Commun. 2024 Feb 15;15(1):1393. doi: 10.1038/s41467-024-45618-z. Nat Commun. 2024. PMID: 38360927 Free PMC article.
-
Kinetic Modeling of Hepatic Metabolism and Simulation of Treatment Effects.Methods Mol Biol. 2024;2769:211-225. doi: 10.1007/978-1-0716-3694-7_16. Methods Mol Biol. 2024. PMID: 38315400
-
Hepatocellular loss of mTOR aggravates tumor burden in nonalcoholic steatohepatitis-related HCC.Neoplasia. 2023 Dec;46:100945. doi: 10.1016/j.neo.2023.100945. Epub 2023 Nov 15. Neoplasia. 2023. PMID: 37976569 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

