Roles of telomeres and telomerase in cancer, and advances in telomerase-_targeted therapies
- PMID: 27323951
- PMCID: PMC4915101
- DOI: 10.1186/s13073-016-0324-x
Roles of telomeres and telomerase in cancer, and advances in telomerase-_targeted therapies
Abstract
Telomeres maintain genomic integrity in normal cells, and their progressive shortening during successive cell divisions induces chromosomal instability. In the large majority of cancer cells, telomere length is maintained by telomerase. Thus, telomere length and telomerase activity are crucial for cancer initiation and the survival of tumors. Several pathways that regulate telomere length have been identified, and genome-scale studies have helped in mapping genes that are involved in telomere length control. Additionally, genomic screening for recurrent human telomerase gene hTERT promoter mutations and mutations in genes involved in the alternative lengthening of telomeres pathway, such as ATRX and DAXX, has elucidated how these genomic changes contribute to the activation of telomere maintenance mechanisms in cancer cells. Attempts have also been made to develop telomere length- and telomerase-based diagnostic tools and anticancer therapeutics. Recent efforts have revealed key aspects of telomerase assembly, intracellular trafficking and recruitment to telomeres for completing DNA synthesis, which may provide novel _targets for the development of anticancer agents. Here, we summarize telomere organization and function and its role in oncogenesis. We also highlight genomic mutations that lead to reactivation of telomerase, and mechanisms of telomerase reconstitution and trafficking that shed light on its function in cancer initiation and tumor development. Additionally, recent advances in the clinical development of telomerase inhibitors, as well as potential novel _targets, will be summarized.
Figures
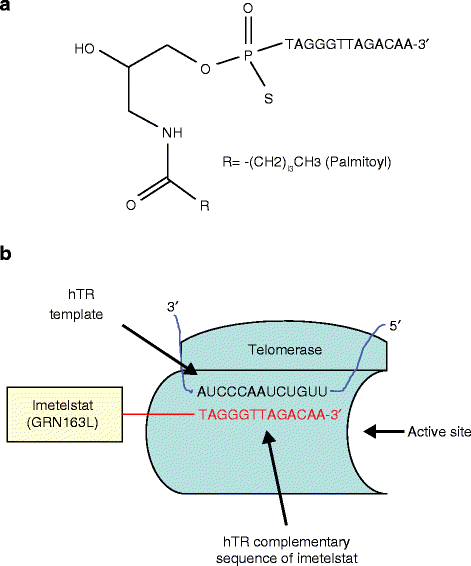
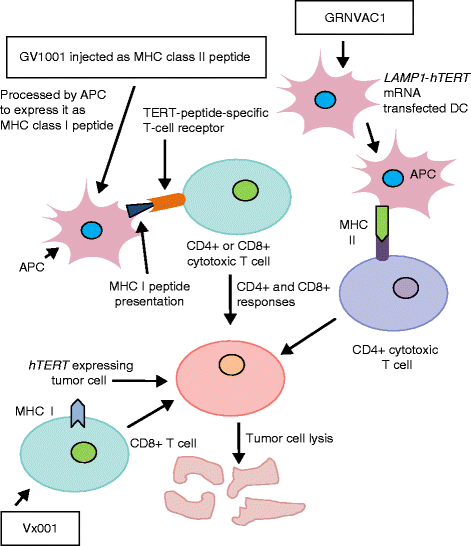
Similar articles
-
Disease mutant analysis identifies a new function of DAXX in telomerase regulation and telomere maintenance.J Cell Sci. 2015 Jan 15;128(2):331-41. doi: 10.1242/jcs.159467. Epub 2014 Nov 21. J Cell Sci. 2015. PMID: 25416818 Free PMC article.
-
Anticancer therapy _targeting telomeres and telomerase : current status.BioDrugs. 2007;21(6):375-85. doi: 10.2165/00063030-200721060-00005. BioDrugs. 2007. PMID: 18020621 Review.
-
Potential Telomere-Related Pharmacological _targets.Curr Top Med Chem. 2020;20(6):458-484. doi: 10.2174/1568026620666200109114339. Curr Top Med Chem. 2020. PMID: 31916516 Review.
-
Telomerase inhibitors as anticancer therapy.Curr Med Chem Anticancer Agents. 2002 Sep;2(5):567-75. doi: 10.2174/1568011023353778. Curr Med Chem Anticancer Agents. 2002. PMID: 12678724 Review.
-
Altered telomeres in tumors with ATRX and DAXX mutations.Science. 2011 Jul 22;333(6041):425. doi: 10.1126/science.1207313. Epub 2011 Jun 30. Science. 2011. PMID: 21719641 Free PMC article.
Cited by
-
Neoadjuvant Chemotherapy Shortens the cfDNA Telomere Length in Breast Cancer Patients.Int J Breast Cancer. 2024 Nov 14;2024:6117394. doi: 10.1155/2024/6117394. eCollection 2024. Int J Breast Cancer. 2024. PMID: 39574517 Free PMC article.
-
Apoptotic effect of thymoquinone on OVCAR3 cells via the P53 and CASP3 activation.Acta Cir Bras. 2024 Nov 8;39:e399224. doi: 10.1590/acb399224. eCollection 2024. Acta Cir Bras. 2024. PMID: 39536185 Free PMC article.
-
Bats as instructive animal models for studying longevity and aging.Ann N Y Acad Sci. 2024 Nov;1541(1):10-23. doi: 10.1111/nyas.15233. Epub 2024 Oct 4. Ann N Y Acad Sci. 2024. PMID: 39365995 Free PMC article. Review.
-
Quercetin Intake and Absolute Telomere Length in Patients with Type 2 Diabetes Mellitus: Novel Findings from a Randomized Controlled Before-and-After Study.Pharmaceuticals (Basel). 2024 Aug 29;17(9):1136. doi: 10.3390/ph17091136. Pharmaceuticals (Basel). 2024. PMID: 39338301 Free PMC article.
-
A First-in-Class High-Throughput Screen to Discover Modulators of the Alternative Lengthening of Telomeres (ALT) Pathway.ACS Pharmacol Transl Sci. 2024 Aug 13;7(9):2799-2819. doi: 10.1021/acsptsci.4c00251. eCollection 2024 Sep 13. ACS Pharmacol Transl Sci. 2024. PMID: 39296266
References
-
- Shay JW. Are short telomeres predictive of advanced cancer? Cancer Discov. 2013;3:1096–8. doi: 10.1158/2159-8290.CD-13-0506. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

