Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies
- PMID: 27766055
- PMCID: PMC5056500
- DOI: 10.1186/s12959-016-0100-6
Platelets and platelet adhesion molecules: novel mechanisms of thrombosis and anti-thrombotic therapies
Abstract
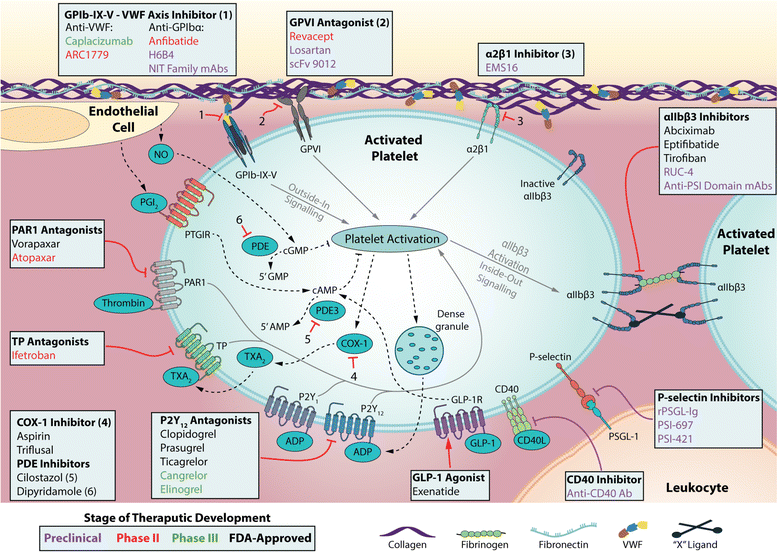
Platelets are central mediators of thrombosis and hemostasis. At the site of vascular injury, platelet accumulation (i.e. adhesion and aggregation) constitutes the first wave of hemostasis. Blood coagulation, initiated by the coagulation cascades, is the second wave of thrombin generation and enhance phosphatidylserine exposure, can markedly potentiate cell-based thrombin generation and enhance blood coagulation. Recently, deposition of plasma fibronectin and other proteins onto the injured vessel wall has been identified as a new "protein wave of hemostasis" that occurs prior to platelet accumulation (i.e. the classical first wave of hemostasis). These three waves of hemostasis, in the event of atherosclerotic plaque rupture, may turn pathogenic, and cause uncontrolled vessel occlusion and thrombotic disorders (e.g. heart attack and stroke). Current anti-platelet therapies have significantly reduced cardiovascular mortality, however, on-treatment thrombotic events, thrombocytopenia, and bleeding complications are still major concerns that continue to motivate innovation and drive therapeutic advances. Emerging evidence has brought platelet adhesion molecules back into the spotlight as _targets for the development of novel anti-thrombotic agents. These potential antiplatelet _targets mainly include the platelet receptors glycoprotein (GP) Ib-IX-V complex, β3 integrins (αIIb subunit and PSI domain of β3 subunit) and GPVI. Numerous efforts have been made aiming to balance the efficacy of inhibiting thrombosis without compromising hemostasis. This mini-review will update the mechanisms of thrombosis and the current state of antiplatelet therapies, and will focus on platelet adhesion molecules and the novel anti-thrombotic therapies that _target them.
Keywords: Anfibatide; GPIbα; GPVI; Hemostasis; Integrins; P-selectin; Stroke; Thrombosis; αIIbβ3.
Figures
Similar articles
-
Platelets are versatile cells: New discoveries in hemostasis, thrombosis, immune responses, tumor metastasis and beyond.Crit Rev Clin Lab Sci. 2016 Dec;53(6):409-30. doi: 10.1080/10408363.2016.1200008. Epub 2016 Jul 22. Crit Rev Clin Lab Sci. 2016. PMID: 27282765 Review.
-
Platelets in thrombosis and hemostasis: old topic with new mechanisms.Cardiovasc Hematol Disord Drug _targets. 2012 Dec;12(2):126-32. doi: 10.2174/1871529x11202020126. Cardiovasc Hematol Disord Drug _targets. 2012. PMID: 23030445 Review.
-
Updated Understanding of Platelets in Thrombosis and Hemostasis: The Roles of Integrin PSI Domains and their Potential as Therapeutic _targets.Cardiovasc Hematol Disord Drug _targets. 2020;20(4):260-273. doi: 10.2174/1871529X20666201001144541. Cardiovasc Hematol Disord Drug _targets. 2020. PMID: 33001021 Review.
-
Platelets in hemostasis and thrombosis: Novel mechanisms of fibrinogen-independent platelet aggregation and fibronectin-mediated protein wave of hemostasis.J Biomed Res. 2015 Oct 30;29(6):437-44. doi: 10.7555/JBR.29.20150121. Online ahead of print. J Biomed Res. 2015. PMID: 26541706 Free PMC article.
-
Platelets, immune-mediated thrombocytopenias, and fetal hemorrhage.Thromb Res. 2016 May;141 Suppl 2:S76-9. doi: 10.1016/S0049-3848(16)30372-3. Thromb Res. 2016. PMID: 27207432 Review.
Cited by
-
Covid-19: The Rollercoaster of Fibrin(Ogen), D-Dimer, Von Willebrand Factor, P-Selectin and Their Interactions with Endothelial Cells, Platelets and Erythrocytes.Int J Mol Sci. 2020 Jul 21;21(14):5168. doi: 10.3390/ijms21145168. Int J Mol Sci. 2020. PMID: 32708334 Free PMC article. Review.
-
Crosstalk between Platelets and SARS-CoV-2: Implications in Thrombo-Inflammatory Complications in COVID-19.Int J Mol Sci. 2023 Sep 15;24(18):14133. doi: 10.3390/ijms241814133. Int J Mol Sci. 2023. PMID: 37762435 Free PMC article. Review.
-
Predictive Role of Blood Cellular Indices and Their Relationship with Endogenous Glycosaminoglycans as Determinants of Inflammatory Biomarkers in Pulmonary Embolism.Clin Appl Thromb Hemost. 2022 Jan-Dec;28:10760296221104801. doi: 10.1177/10760296221104801. Clin Appl Thromb Hemost. 2022. PMID: 35733366 Free PMC article.
-
Inhibition of PCSK9 Protects against Cerebral Ischemia‒Reperfusion Injury via Attenuating Microcirculatory Dysfunction.Neurochem Res. 2024 Nov 16;50(1):10. doi: 10.1007/s11064-024-04272-z. Neurochem Res. 2024. PMID: 39548030
-
Crosstalk Between Platelets and Microbial Pathogens.Front Immunol. 2020 Aug 7;11:1962. doi: 10.3389/fimmu.2020.01962. eCollection 2020. Front Immunol. 2020. PMID: 32849656 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

