Complementary Split-Ring Resonator-Loaded Microfluidic Ethanol Chemical Sensor
- PMID: 27801842
- PMCID: PMC5134461
- DOI: 10.3390/s16111802
Complementary Split-Ring Resonator-Loaded Microfluidic Ethanol Chemical Sensor
Abstract
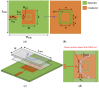
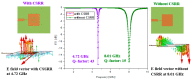
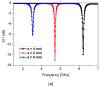
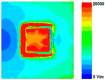
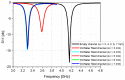
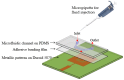
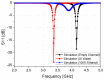
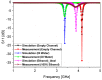
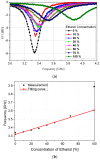
In this paper, a complementary split-ring resonator (CSRR)-loaded patch is proposed as a microfluidic ethanol chemical sensor. The primary objective of this chemical sensor is to detect ethanol's concentration. First, two tightly coupled concentric CSRRs loaded on a patch are realized on a Rogers RT/Duroid 5870 substrate, and then a microfluidic channel engraved on polydimethylsiloxane (PDMS) is integrated for ethanol chemical sensor applications. The resonant frequency of the structure before loading the microfluidic channel is 4.72 GHz. After loading the microfluidic channel, the 550 MHz shift in the resonant frequency is ascribed to the dielectric perturbation phenomenon when the ethanol concentration is varied from 0% to 100%. In order to assess the sensitivity range of our proposed sensor, various concentrations of ethanol are tested and analyzed. Our proposed sensor exhibits repeatability and successfully detects 10% ethanol as verified by the measurement set-up. It has created headway to a miniaturized, non-contact, low-cost, reliable, reusable, and easily fabricated design using extremely small liquid volumes.
Keywords: complementary split ring resonator (CSRR); ethanol sensor; microfluidic channel; patch.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Quarter-mode spoof plasmonic resonator for a microfluidic chemical sensor.Appl Opt. 2018 Oct 1;57(28):8472-8477. doi: 10.1364/AO.57.008472. Appl Opt. 2018. PMID: 30461805
-
Stretchable Complementary Split Ring Resonator (CSRR)-Based Radio Frequency (RF) Sensor for Strain Direction and Level Detection.Sensors (Basel). 2016 Oct 11;16(10):1667. doi: 10.3390/s16101667. Sensors (Basel). 2016. PMID: 27727173 Free PMC article.
-
Frequency-Switchable Microfluidic CSRR-Loaded QMSIW Band-Pass Filter Using a Liquid Metal Alloy.Sensors (Basel). 2017 Mar 28;17(4):699. doi: 10.3390/s17040699. Sensors (Basel). 2017. PMID: 28350355 Free PMC article.
-
Design of a High-Sensitivity Microstrip Patch Sensor Antenna Loaded with a Defected Ground Structure Based on a Complementary Split Ring Resonator.Sensors (Basel). 2020 Dec 10;20(24):7064. doi: 10.3390/s20247064. Sensors (Basel). 2020. PMID: 33321740 Free PMC article.
-
A Compact and Low-Profile Curve-Feed Complementary Split-Ring Resonator Microwave Sensor for Solid Material Detection.Micromachines (Basel). 2023 Feb 3;14(2):384. doi: 10.3390/mi14020384. Micromachines (Basel). 2023. PMID: 36838083 Free PMC article. Review.
Cited by
-
Review of Recent Microwave Planar Resonator-Based Sensors: Techniques of Complex Permittivity Extraction, Applications, Open Challenges and Future Research Directions.Sensors (Basel). 2021 Mar 24;21(7):2267. doi: 10.3390/s21072267. Sensors (Basel). 2021. PMID: 33804904 Free PMC article. Review.
-
Meander-Line Slot-Loaded High-Sensitivity Microstrip Patch Sensor Antenna for Relative Permittivity Measurement.Sensors (Basel). 2019 Oct 27;19(21):4660. doi: 10.3390/s19214660. Sensors (Basel). 2019. PMID: 31717843 Free PMC article.
-
TM02 Quarter-Mode Substrate-Integrated Waveguide Resonator for Dual Detection of Chemicals.Sensors (Basel). 2018 Jun 18;18(6):1964. doi: 10.3390/s18061964. Sensors (Basel). 2018. PMID: 29912164 Free PMC article.
-
Highly Sensitive Microwave Sensors Based on Open Complementary Square Split-Ring Resonator for Sensing Liquid Materials.Sensors (Basel). 2024 Mar 13;24(6):1840. doi: 10.3390/s24061840. Sensors (Basel). 2024. PMID: 38544102 Free PMC article.
-
A Microwave Microfluidic Sensor Based on a Dual-Mode Resonator for Dual-Sensing Applications.Sensors (Basel). 2017 Nov 24;17(12):2713. doi: 10.3390/s17122713. Sensors (Basel). 2017. PMID: 29186767 Free PMC article.
References
-
- Timmer B., Olthuis W., Berg A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005;107:666–677. doi: 10.1016/j.snb.2004.11.054. - DOI
-
- Cheng X.L., Zhao H., Huo L.H., Gao S., Zhao J.G. ZnO nanoparticulate thin film: Preparation, characterization and gas-sensing property. Sens. Actuators B Chem. 2004;102:248–252. doi: 10.1016/j.snb.2004.04.080. - DOI
-
- Forleo A., Francioso L., Capone S., Siciliano P., Lommens P., Hens Z. Synthesis and gas sensing properties of ZnO quantum dots. Sens. Actuators B Chem. 2010;146:111–115. doi: 10.1016/j.snb.2010.02.059. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

