Ubiquitin Ligase NEDD4 Regulates PPARγ Stability and Adipocyte Differentiation in 3T3-L1 Cells
- PMID: 27917940
- PMCID: PMC5137149
- DOI: 10.1038/srep38550
Ubiquitin Ligase NEDD4 Regulates PPARγ Stability and Adipocyte Differentiation in 3T3-L1 Cells
Abstract
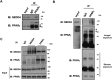
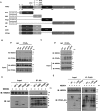
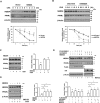
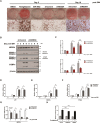
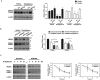
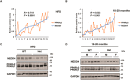
Peroxisome proliferator-activated receptor-γ (PPARγ) is a ligand-activated nuclear receptor which controls lipid and glucose metabolism. It is also the master regulator of adipogenesis. In adipocytes, ligand-dependent PPARγ activation is associated with proteasomal degradation; therefore, regulation of PPARγ degradation may modulate its transcriptional activity. Here, we show that neural precursor cell expressed developmentally down-regulated protein 4 (NEDD4), an E3 ubiquitin ligase, interacts with the hinge and ligand binding domains of PPARγ and is a bona fide E3 ligase for PPARγ. NEDD4 increases PPARγ stability through the inhibition of its proteasomal degradation. Knockdown of NEDD4 in 3T3-L1 adipocytes reduces PPARγ protein levels and suppresses adipocyte conversion. PPARγ correlates positively with NEDD4 in obese adipose tissue. Together, these findings support NEDD4 as a novel regulator of adipogenesis by modulating the stability of PPARγ.
Figures

Similar articles
-
The E3 ubiquitin ligase TRIM25 regulates adipocyte differentiation via proteasome-mediated degradation of PPARγ.Exp Mol Med. 2018 Oct 15;50(10):1-11. doi: 10.1038/s12276-018-0162-6. Exp Mol Med. 2018. PMID: 30323259 Free PMC article.
-
Aryl hydrocarbon receptor (AhR) regulates adipocyte differentiation by assembling CRL4B ubiquitin ligase to _target PPARγ for proteasomal degradation.J Biol Chem. 2019 Nov 29;294(48):18504-18515. doi: 10.1074/jbc.RA119.009282. Epub 2019 Oct 25. J Biol Chem. 2019. PMID: 31653699 Free PMC article.
-
C-terminus of HSC70-Interacting Protein (CHIP) Inhibits Adipocyte Differentiation via Ubiquitin- and Proteasome-Mediated Degradation of PPARγ.Sci Rep. 2017 Jan 6;7:40023. doi: 10.1038/srep40023. Sci Rep. 2017. PMID: 28059128 Free PMC article.
-
The E3 ubiquitin ligase TRIM23 regulates adipocyte differentiation via stabilization of the adipogenic activator PPARγ.Elife. 2015 Apr 23;4:e05615. doi: 10.7554/eLife.05615. Elife. 2015. PMID: 25905670 Free PMC article.
-
Coordinate functional regulation between microsomal prostaglandin E synthase-1 (mPGES-1) and peroxisome proliferator-activated receptor γ (PPARγ) in the conversion of white-to-brown adipocytes.J Biol Chem. 2013 Sep 27;288(39):28230-42. doi: 10.1074/jbc.M113.468603. Epub 2013 Aug 13. J Biol Chem. 2013. PMID: 23943621 Free PMC article.
Cited by
-
The E3 ubiquitin ligase TRIM25 regulates adipocyte differentiation via proteasome-mediated degradation of PPARγ.Exp Mol Med. 2018 Oct 15;50(10):1-11. doi: 10.1038/s12276-018-0162-6. Exp Mol Med. 2018. PMID: 30323259 Free PMC article.
-
Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors.Inflamm Res. 2019 Jun;68(6):443-458. doi: 10.1007/s00011-019-01231-1. Epub 2019 Mar 29. Inflamm Res. 2019. PMID: 30927048 Free PMC article. Review.
-
Lysine 222 in PPAR γ1 functions as the key site of MuRF2-mediated ubiquitination modification.Sci Rep. 2023 Feb 3;13(1):1999. doi: 10.1038/s41598-023-28905-5. Sci Rep. 2023. PMID: 36737649 Free PMC article.
-
Insufficient ablation induces E3-ligase Nedd4 to promote hepatocellular carcinoma progression by tuning TGF-β signaling.Oncogene. 2022 Jun;41(23):3197-3209. doi: 10.1038/s41388-022-02334-6. Epub 2022 Apr 30. Oncogene. 2022. PMID: 35501461
-
Curcumin prevents obesity by _targeting TRAF4-induced ubiquitylation in m6 A-dependent manner.EMBO Rep. 2021 May 5;22(5):e52146. doi: 10.15252/embr.202052146. Epub 2021 Apr 20. EMBO Rep. 2021. PMID: 33880847 Free PMC article.
References
-
- Delerive P., Fruchart J. C. & Staels B. Peroxisome proliferator-activated receptors in inflammation control. J. Endocrinol. 169, 453–459 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

