Brain-to-stomach transfer of α-synuclein via vagal preganglionic projections
- PMID: 28012041
- PMCID: PMC5326583
- DOI: 10.1007/s00401-016-1661-y
Brain-to-stomach transfer of α-synuclein via vagal preganglionic projections
Abstract
Detection of α-synuclein lesions in peripheral tissues is a feature of human synucleinopathies of likely pathogenetic relevance and bearing important clinical implications. Experiments were carried out to elucidate the relationship between α-synuclein accumulation in the brain and in peripheral organs, and to identify potential pathways involved in long-distance protein transfer. Results of this in vivo study revealed a route-specific transmission of α-synuclein from the rat brain to the stomach. Following _targeted midbrain overexpression of human α-synuclein, the exogenous protein was capable of reaching the gastric wall where it was accumulated into preganglionic vagal terminals. This brain-to-stomach connection likely involved intra- and inter-neuronal transfer of non-fibrillar α-synuclein that first reached the medulla oblongata, then gained access into cholinergic neurons of the dorsal motor nucleus of the vagus nerve and finally traveled via efferent fibers of these neurons contained within the vagus nerve. Data also showed a particular propensity of vagal motor neurons and efferents to accrue α-synuclein and deliver it to peripheral tissues; indeed, following its midbrain overexpression, human α-synuclein was detected within gastric nerve endings of visceromotor but not viscerosensory vagal projections. Thus, the dorsal motor nucleus of the vagus nerve represents a key relay center for central-to-peripheral α-synuclein transmission, and efferent vagal fibers may act as unique conduits for protein transfer. The presence of α-synuclein in peripheral tissues could reflect, at least in some synucleinopathy patients, an ongoing pathological process that originates within the brain and, from there, reaches distant organs innervated by motor vagal projections.
Keywords: Adeno-associated virus; Enteric nervous system; Parkinson’s disease; Rat; Synucleinopathies; Vagus nerve.
Figures



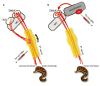
Similar articles
-
Overexpression-Induced α-Synuclein Brain Spreading.Neurotherapeutics. 2023 Jan;20(1):83-96. doi: 10.1007/s13311-022-01332-6. Epub 2022 Dec 13. Neurotherapeutics. 2023. PMID: 36512255 Free PMC article. Review.
-
Alpha-synuclein-immunopositive myenteric neurons and vagal preganglionic terminals: autonomic pathway implicated in Parkinson's disease?Neuroscience. 2008 May 15;153(3):733-50. doi: 10.1016/j.neuroscience.2008.02.074. Epub 2008 Mar 18. Neuroscience. 2008. PMID: 18407422 Free PMC article.
-
Brain propagation of transduced α-synuclein involves non-fibrillar protein species and is enhanced in α-synuclein null mice.Brain. 2016 Mar;139(Pt 3):856-70. doi: 10.1093/brain/awv376. Epub 2015 Dec 30. Brain. 2016. PMID: 26719384
-
Caudo-rostral brain spreading of α-synuclein through vagal connections.EMBO Mol Med. 2013 Jul;5(7):1119-27. doi: 10.1002/emmm.201302475. Epub 2013 May 23. EMBO Mol Med. 2013. PMID: 23703938 Free PMC article.
-
Review: Sporadic Parkinson's disease: development and distribution of α-synuclein pathology.Neuropathol Appl Neurobiol. 2016 Feb;42(1):33-50. doi: 10.1111/nan.12298. Neuropathol Appl Neurobiol. 2016. PMID: 26662475 Review.
Cited by
-
Overexpression-Induced α-Synuclein Brain Spreading.Neurotherapeutics. 2023 Jan;20(1):83-96. doi: 10.1007/s13311-022-01332-6. Epub 2022 Dec 13. Neurotherapeutics. 2023. PMID: 36512255 Free PMC article. Review.
-
Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats.Acta Neuropathol. 2019 Oct;138(4):535-550. doi: 10.1007/s00401-019-02040-w. Epub 2019 Jun 26. Acta Neuropathol. 2019. PMID: 31254094 Free PMC article.
-
Molecular Communication Between Neuronal Networks and Intestinal Epithelial Cells in Gut Inflammation and Parkinson's Disease.Front Med (Lausanne). 2021 Jul 22;8:655123. doi: 10.3389/fmed.2021.655123. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34368179 Free PMC article. Review.
-
Neuropathological Staging of Brain Pathology in Sporadic Parkinson's disease: Separating the Wheat from the Chaff.J Parkinsons Dis. 2017;7(s1):S71-S85. doi: 10.3233/JPD-179001. J Parkinsons Dis. 2017. PMID: 28282810 Free PMC article. Review.
-
Viruses and Multiple Sclerosis: From Mechanisms and Pathways to Translational Research Opportunities.Mol Neurobiol. 2017 Jul;54(5):3911-3923. doi: 10.1007/s12035-017-0530-6. Epub 2017 Apr 28. Mol Neurobiol. 2017. PMID: 28455696 Review.
References
-
- Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White CL, III, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119:689–702. doi: 10.1007/s00401-010-0664-3. - DOI - PMC - PubMed
-
- Berthoud HR, Jedrzejewska A, Powley TL. Simultaneous labeling of vagal innervation of the gut and afferent projections from the visceral forebrain with dil injected into the dorsal vagal complex in the rat. J Comp Neurol. 1990;301:65–79. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

