Bezafibrate ameliorates diabetes via reduced steatosis and improved hepatic insulin sensitivity in diabetic TallyHo mice
- PMID: 28271032
- PMCID: PMC5323884
- DOI: 10.1016/j.molmet.2016.12.007
Bezafibrate ameliorates diabetes via reduced steatosis and improved hepatic insulin sensitivity in diabetic TallyHo mice
Abstract
Objective: Recently, we have shown that Bezafibrate (BEZ), the pan-PPAR (peroxisome proliferator-activated receptor) activator, ameliorated diabetes in insulin deficient streptozotocin treated diabetic mice. In order to study whether BEZ can also improve glucose metabolism in a mouse model for fatty liver and type 2 diabetes, the drug was applied to TallyHo mice.
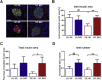
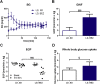
Methods: TallyHo mice were divided into an early (ED) and late (LD) diabetes progression group and both groups were treated with 0.5% BEZ (BEZ group) or standard diet (SD group) for 8 weeks. We analyzed plasma parameters, pancreatic beta-cell morphology, and mass as well as glucose metabolism of the BEZ-treated and control mice. Furthermore, liver fat content and composition as well as hepatic gluconeogenesis and mitochondrial mass were determined.
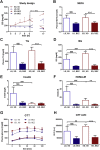
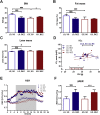
Results: Plasma lipid and glucose levels were markedly reduced upon BEZ treatment, which was accompanied by elevated insulin sensitivity index as well as glucose tolerance, respectively. BEZ increased islet area in the pancreas. Furthermore, BEZ treatment improved energy expenditure and metabolic flexibility. In the liver, BEZ ameliorated steatosis, modified lipid composition and increased mitochondrial mass, which was accompanied by reduced hepatic gluconeogenesis.
Conclusions: Our data showed that BEZ ameliorates diabetes probably via reduced steatosis, enhanced hepatic mitochondrial mass, improved metabolic flexibility and elevated hepatic insulin sensitivity in TallyHo mice, suggesting that BEZ treatment could be beneficial for patients with NAFLD and impaired glucose metabolism.
Keywords: BEZ, Bezafibrate; BG, blood glucose; Bezafibrate; ED, early onset of diabetes; EM, electron microscopy; FA, fatty acid; Glucose metabolism; HOMA-IR, homeostatic model assessment of insulin resistance; Insulin resistance; LD, late onset of diabetes; Lipid metabolism; NAFLD; NAFLD, non-alcoholic fatty liver disease; NEFA, non-esterified fatty acid; PPAR, peroxisome proliferator-activated receptor; RER, respiratory exchange ratios; SD, standard diet; T2D, type 2 diabetes; TG, triglyceride; qNMR, quantitative nuclear magnetic resonance.
Figures

Similar articles
-
Bezafibrate Improves Insulin Sensitivity and Metabolic Flexibility in STZ-Induced Diabetic Mice.Diabetes. 2016 Sep;65(9):2540-52. doi: 10.2337/db15-1670. Epub 2016 Jun 9. Diabetes. 2016. PMID: 27284107
-
Bezafibrate on lipids and glucose metabolism in obese diabetic Otsuka Long-Evans Tokushima fatty rats.Metabolism. 2004 Apr;53(4):405-13. doi: 10.1016/j.metabol.2003.10.006. Metabolism. 2004. PMID: 15045684
-
Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects.Mol Cell Endocrinol. 2013 Aug 25;376(1-2):70-80. doi: 10.1016/j.mce.2013.06.014. Epub 2013 Jun 18. Mol Cell Endocrinol. 2013. PMID: 23791844
-
Balanced pan-PPAR activator bezafibrate in combination with statin: comprehensive lipids control and diabetes prevention?Cardiovasc Diabetol. 2012 Nov 14;11:140. doi: 10.1186/1475-2840-11-140. Cardiovasc Diabetol. 2012. PMID: 23150952 Free PMC article. Review.
-
Dual and pan-peroxisome proliferator-activated receptors (PPAR) co-agonism: the bezafibrate lessons.Cardiovasc Diabetol. 2005 Sep 16;4:14. doi: 10.1186/1475-2840-4-14. Cardiovasc Diabetol. 2005. PMID: 16168052 Free PMC article. Review.
Cited by
-
Mitochondrial oxidative phosphorylation is impaired in TALLYHO mice, a new obesity and type 2 diabetes animal model.Int J Biochem Cell Biol. 2019 Nov;116:105616. doi: 10.1016/j.biocel.2019.105616. Epub 2019 Sep 19. Int J Biochem Cell Biol. 2019. PMID: 31542429 Free PMC article.
-
Peroxisome Proliferator-Activated Receptors and Their Agonists in Nonalcoholic Fatty Liver Disease.J Clin Exp Hepatol. 2019 Nov-Dec;9(6):731-739. doi: 10.1016/j.jceh.2019.06.004. Epub 2019 Jul 2. J Clin Exp Hepatol. 2019. PMID: 31889755 Free PMC article. Review.
-
Long-Term Use of Statins Lowering the Risk of Rehospitalization Caused by Ischemic Stroke Among Middle-Aged Hyperlipidemic Patients: A Population-Based Study.Front Pharmacol. 2021 Oct 18;12:741094. doi: 10.3389/fphar.2021.741094. eCollection 2021. Front Pharmacol. 2021. PMID: 34733160 Free PMC article.
-
A Comprehensive Study of High Cholesterol Diet-Induced Larval Zebrafish Model: A Short-Time In Vivo Screening Method for Non-Alcoholic Fatty Liver Disease Drugs.Int J Biol Sci. 2019 Mar 10;15(5):973-983. doi: 10.7150/ijbs.30013. eCollection 2019. Int J Biol Sci. 2019. PMID: 31182918 Free PMC article.
-
Epigallocatechin gallate (EGCG) reduces the intensity of pancreatic amyloid fibrils in human islet amyloid polypeptide (hIAPP) transgenic mice.Sci Rep. 2018 Jan 18;8(1):1116. doi: 10.1038/s41598-017-18807-8. Sci Rep. 2018. PMID: 29348618 Free PMC article.
References
-
- Matsui H., Okumura K., Kawakami K., Hibino M., Toki Y., Ito T. Improved insulin sensitivity by bezafibrate in rats: relationship to fatty acid composition of skeletal-muscle triglycerides. Diabetes. 1997;46:348–353. - PubMed
-
- Jones I.R., Swai A., Taylor R., Miller M., Laker M.F., Alberti K.G. Lowering of plasma glucose concentrations with bezafibrate in patients with moderately controlled NIDDM. Diabetes Care. 1990;13:855–863. - PubMed
-
- Franko A., Huypens P., Neschen S., Irmler M., Rozman J., Rathkolb B. Bezafibrate improves insulin sensitivity and metabolic flexibility in STZ-induced diabetic mice. Diabetes. 2016;65:2540–2552. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

